Question: A new process is considered for producing ethylene (C2H4) gas from propane (C3H8) gas at atmospheric pressure using the reaction C3H8(g)+2O2(g)C2H4(g)+CO2(g)+2H2O(g) The products leave the
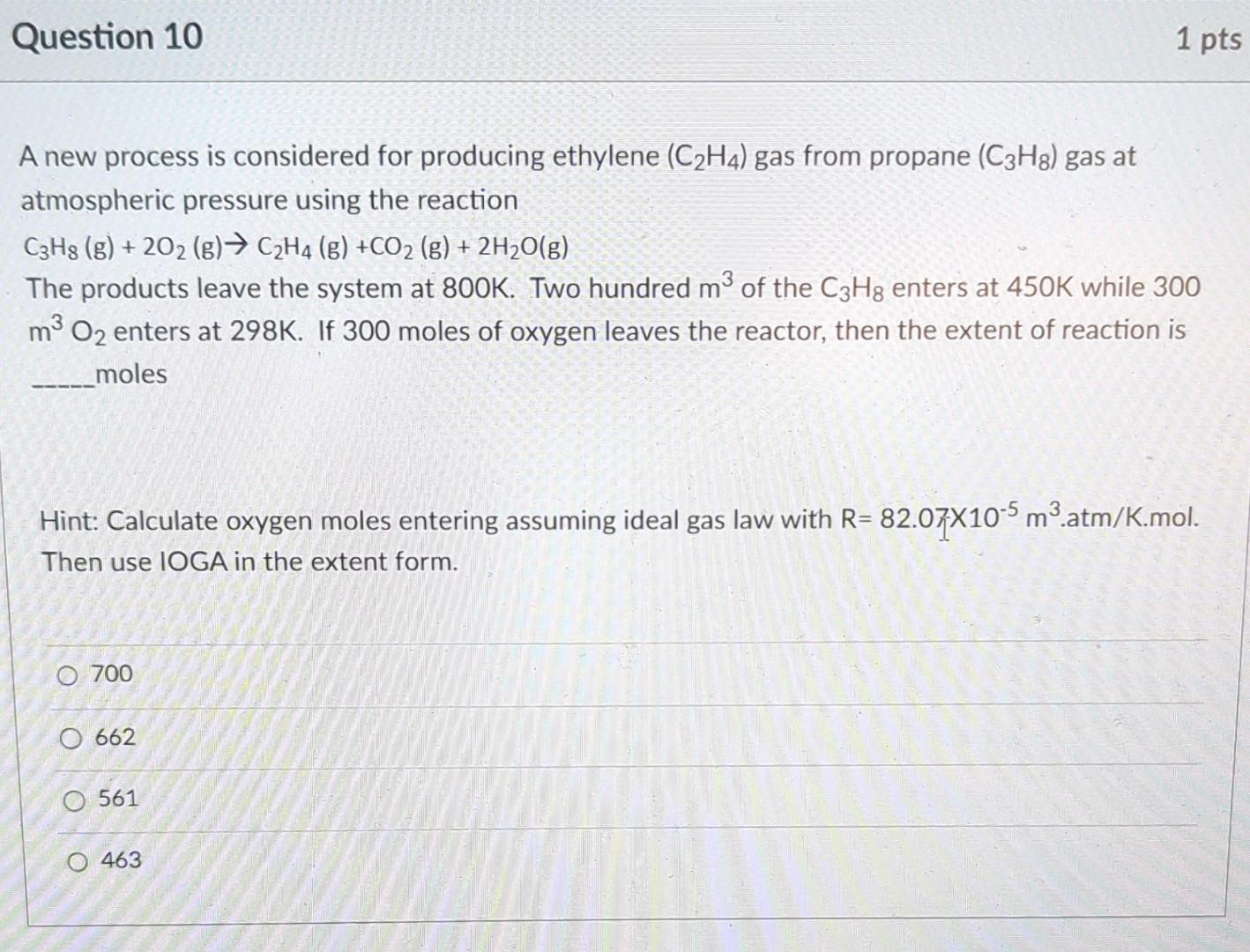
A new process is considered for producing ethylene (C2H4) gas from propane (C3H8) gas at atmospheric pressure using the reaction C3H8(g)+2O2(g)C2H4(g)+CO2(g)+2H2O(g) The products leave the system at 800K. Two hundred m3 of the C3H8 enters at 450K while 300 m3O2 enters at 298K. If 300 moles of oxygen leaves the reactor, then the extent of reaction is moles Hint: Calculate oxygen moles entering assuming ideal gas law with R=82.07105m3.atm/K.mol. Then use IOGA in the extent form. 700 662 561 463
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


