Question: A one - component material has three possible structures: , , and . At high temperatures, the system is . If it is cooled slowly,
A onecomponent material has three possible structures: and At high
temperatures, the system is If it is cooled slowly, it transforms to at some
temperature below the transformation temperature, ie
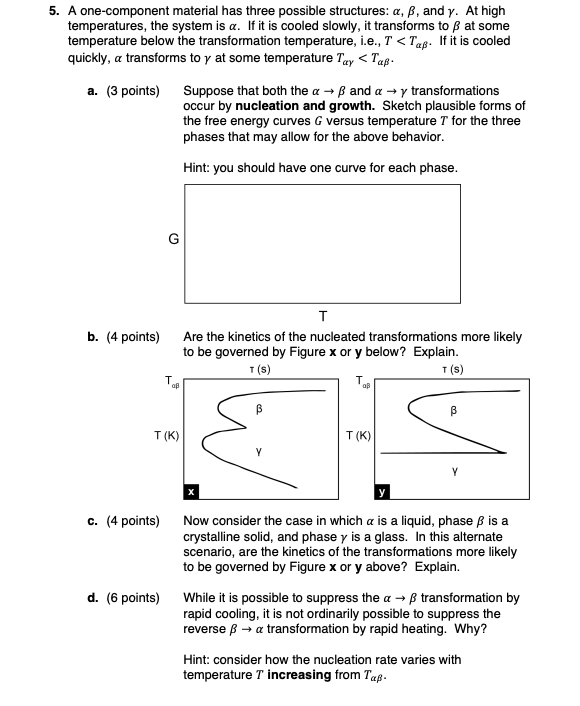
points Suppose that both the and transformations
occur nucleation and growth. Sketch plausible forms
the free energy curves versus temperature for the three
phases that may allow for the above behavior.
Hint: you should have one curve for each phase.
points Are the kinetics the nucleated transformations more likely
governed Figure below? Explain.
points
points
Now consider the case which a liquid, phase
crystalline solid, and phase a glass. this alternate
scenario, are the kinetics the transformations more likely
governed Figure above? Explain.
While possible suppress the transformation
rapid cooling, not ordinarily possible suppress the
reverse transformation rapid heating. Why?
Hint: consider how the nucleation rate varies with
temperature increasing from cooled
quickly, transforms some temperature
points Suppose that both the and transformations
occur nucleation and growth. Sketch plausible forms
the free energy curves versus temperature for the three
phases that may allow for the above behavior.
Hint: you should have one curve for each phase.
points Are the kinetics the nucleated transformations more likely
governed Figure below? Explain.
points
points
Now consider the case which a liquid, phase
crystalline solid, and phase a glass. this alternate
scenario, are the kinetics the transformations more likely
governed Figure above? Explain.
While possible suppress the transformation
rapid cooling, not ordinarily possible suppress the
reverse transformation rapid heating. Why?
Hint: consider how the nucleation rate varies with
temperature increasing from
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


