Question: A phase diagram for the n-hexane + methanol system at 1 atmosphere is shown below. It displays both vapor-liquid equilibrium as well as liquid-liquid equilibrium.
A phase diagram for the n-hexane + methanol system at 1 atmosphere is shown below. It displays both vapor-liquid equilibrium as well as liquid-liquid equilibrium. a. Label the regions VLE, LLE, Single phase liquid and V b. List the boiling points of pure methanol and pure hexane. T c. How many critical points can be identified in this diagram? List their temperatures and compositions. d. A mixture containing 2 mols of methanol and 8 mols of hexane are held at 270K. What is the composition of the methanol-rich phase? What is the total number of moles in the methanol-rich phase? What is the number of moles of methanol in the methanol-rich phase? e. Explain in terms of intermolecular attractions and repulsion why this system shows both a minimum-boiling azeotrope as well as liquid-liquid equilibrium. f. This diagram does not show three-phase equilibrium, i.e. a temperature at which two liquid phases and one vapor phase coexist. Could lowering or raising the pressure cause a three-phase equilibrium to appear? Explain your answer qualitatively, explaining why you would raise or lower the pressure.
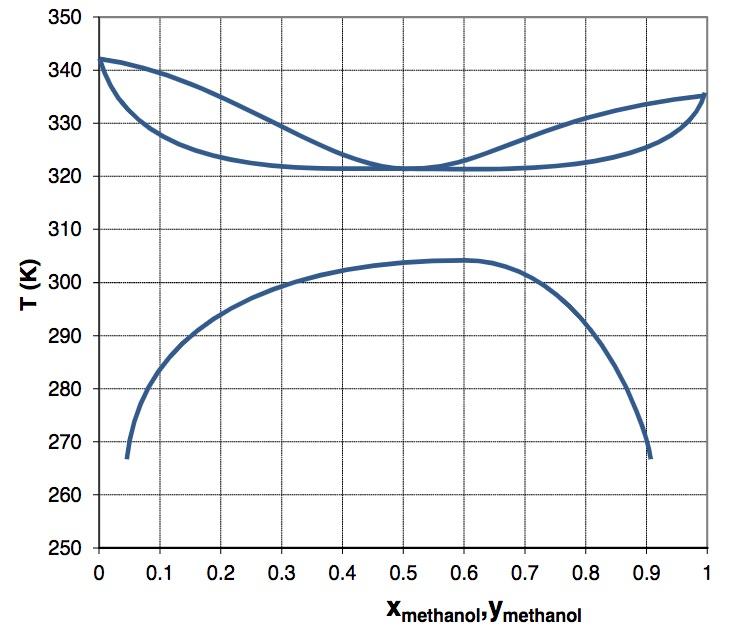
350 340 330 320 310 E 300 290 280 270 260 250 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1 XmethanolsYmethanol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


