Question: A pitcher filter is designed to remove lead cation (Pb+, 7 ug/L) from the contaminated tap water. The tap water also contains the following
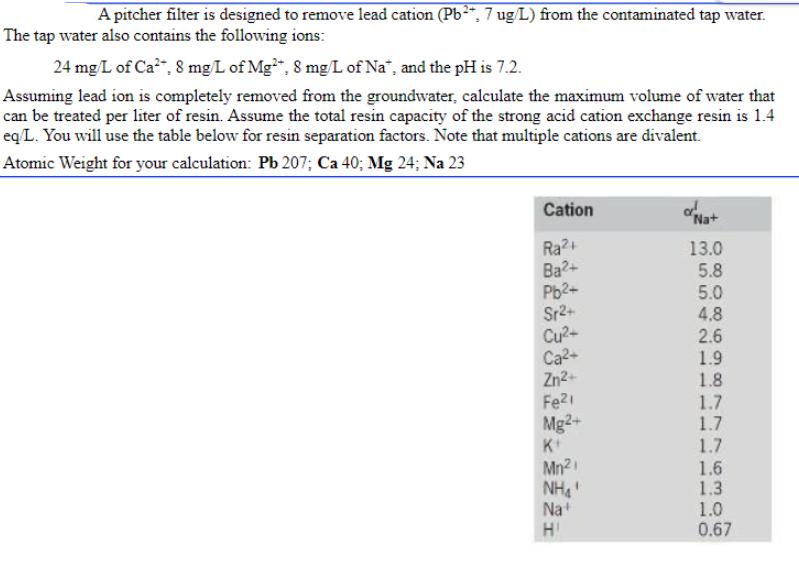
A pitcher filter is designed to remove lead cation (Pb+, 7 ug/L) from the contaminated tap water. The tap water also contains the following ions: 24 mg/L of Ca, 8 mg/L of Mg2+, 8 mg/L of Na", and the pH is 7.2. Assuming lead ion is completely removed from the groundwater, calculate the maximum volume of water that can be treated per liter of resin. Assume the total resin capacity of the strong acid cation exchange resin is 1.4 eq/L. You will use the table below for resin separation factors. Note that multiple cations are divalent. Atomic Weight for your calculation: Pb 207; Ca 40; Mg 24; Na 23 Cation Ra2+ Ba2+ Pb+ Sr+ Cu+ Ca+ Zn2+ Fe1 Mg2+ K Mn2i NH' Na+ HI "Na+ 13.0 5.8 5.0 4.8 2.6 1.9 1.8 1.7 1.7 1.7 1.6 1.3 1.0 0.67
Step by Step Solution
3.34 Rating (145 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


