Question: a. Plot the appropriate polarization curves for the following half cell reactions and determine the corrosion potential and corrosion rate (current density) assuming activation
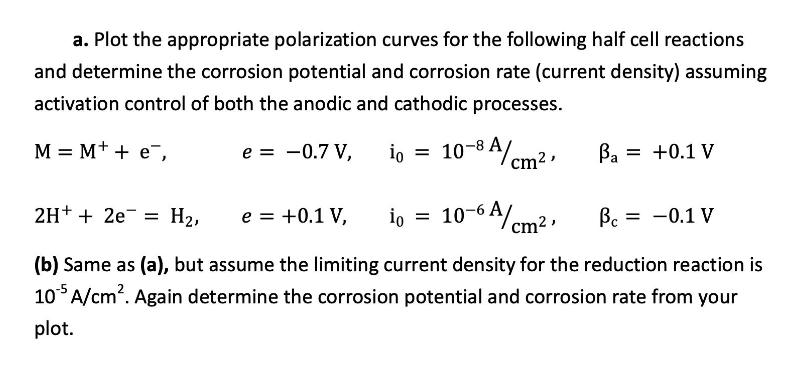
a. Plot the appropriate polarization curves for the following half cell reactions and determine the corrosion potential and corrosion rate (current density) assuming activation control of both the anodic and cathodic processes. 10-8A/cm, M = M+ + e, e = -0.7 V, io = io 10-6A/cm, = Ba = +0.1 V 2H+ + 2e H, e = +0.1 V, (b) Same as (a), but assume the limiting current density for the reduction reaction is 105 A/cm. Again determine the corrosion potential and corrosion rate from your plot. = -0.1 V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


