Question: A process at constant I and P can be described as spontaneous if ?G <0 and nonspontaneous if ?G> 0. Over what range of
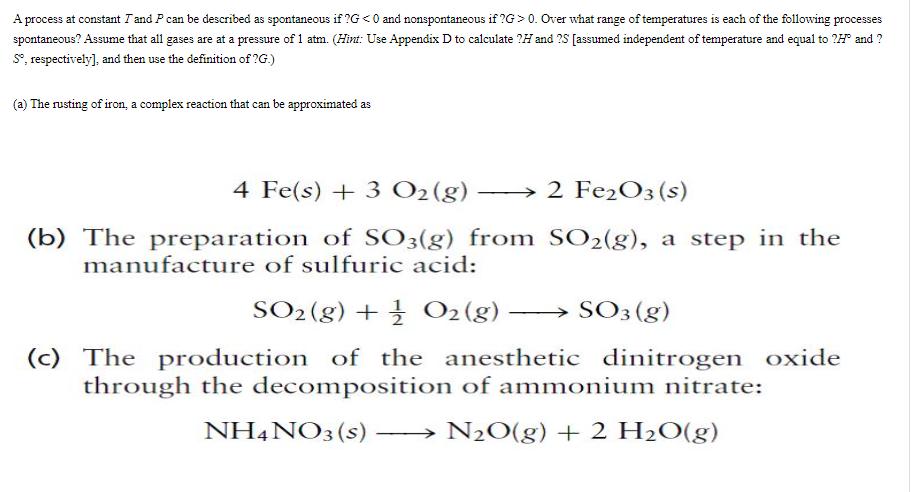
A process at constant I and P can be described as spontaneous if ?G 0. Over what range of temperatures is each of the following processes spontaneous? Assume that all gases are at a pressure of 1 atm. (Hint: Use Appendix D to calculate ? and ?S [assumed independent of temperature and equal to ?H" and ? S, respectively], and then use the definition of ?G.) (a) The rusting of iron, a complex reaction that can be approximated as 4 Fe(s) + 3 O2 (g) (b) The preparation of SO3(g) from manufacture of sulfuric acid: SO2(g) + O2(g) SO3 (g) (c) The production of the anesthetic dinitrogen oxide through the decomposition of ammonium nitrate: NH4NO3 (s) NO(g) + 2 HO(g) 2 Fe2O3 (s) SO2(g), a step in the
Step by Step Solution
3.45 Rating (161 Votes )
There are 3 Steps involved in it
To determine the temperature range over which each process is spontaneous we can use the Gibbs free ... View full answer

Get step-by-step solutions from verified subject matter experts


