Question: A propane BBQ combusts propane as shown below: C3H8 (g) + 5 O2(g) 3 CO2 (g) + 4 HO (1) ArH=-2220 kJ/mol What mass
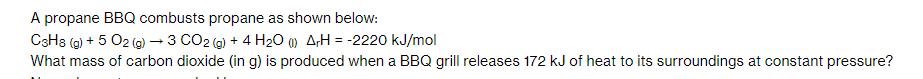
A propane BBQ combusts propane as shown below: C3H8 (g) + 5 O2(g) 3 CO2 (g) + 4 HO (1) ArH=-2220 kJ/mol What mass of carbon dioxide (in g) is produced when a BBQ grill releases 172 kJ of heat to its surroundings at constant pressure?
Step by Step Solution
There are 3 Steps involved in it
Calculating the mass of carbon dioxide produced Step 1 Determine the enthalpy change per gram of CO2 ... View full answer

Get step-by-step solutions from verified subject matter experts


