Question: A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded
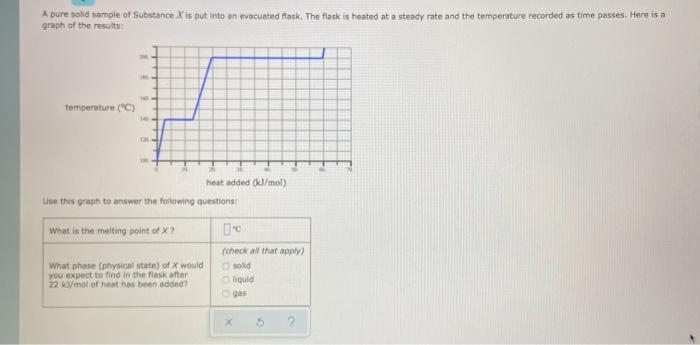
A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results temperature ("C) heat indded (I/mol Use this graph to answer the following questions: What is the melting point of X? What phase (physical state of X would you expect to find in the flask after 22 mol of heat has been added? (check all that apply) sold liquid 5 A pure solid sample of Substance X is put into an evacuated flask. The flask is heated at a steady rate and the temperature recorded as time passes. Here is a graph of the results temperature ("C) heat indded (I/mol Use this graph to answer the following questions: What is the melting point of X? What phase (physical state of X would you expect to find in the flask after 22 mol of heat has been added? (check all that apply) sold liquid 5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


