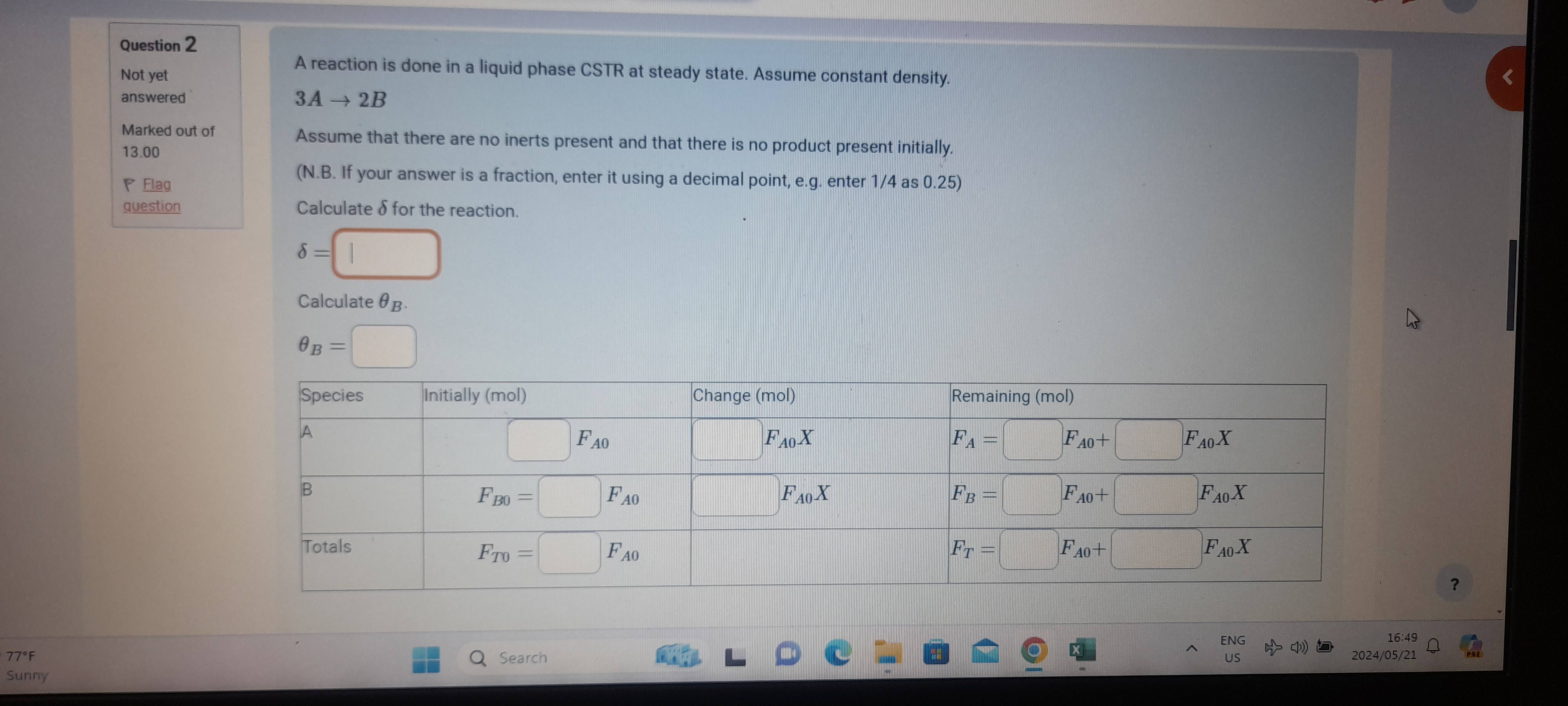
Question: A reaction is done in a liquid phase CSTR at steady state. Assume constant density. 3 A 2 B Assume that there are no inerts
A reaction is done in a liquid phase CSTR at steady state. Assume constant density.
Assume that there are no inerts present and that there is no product present initially.
NB If your answer is a fraction, enter it using a decimal point, eg enter as
Calculate for the reaction.
Calculate
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


