Question: A' Read aloud | Draw 1. a. (i) Draw the structure of each of the following alkenes. . trans-2,3-Dibromobut-2-ene 4-Chloro-1-methylcyclopent-1-ene (3Z)-Hept-3-en-4-ol . (22,4E)-3-Ethyl-2-fluoro-4-methylhexa-2,4-diene (11) State
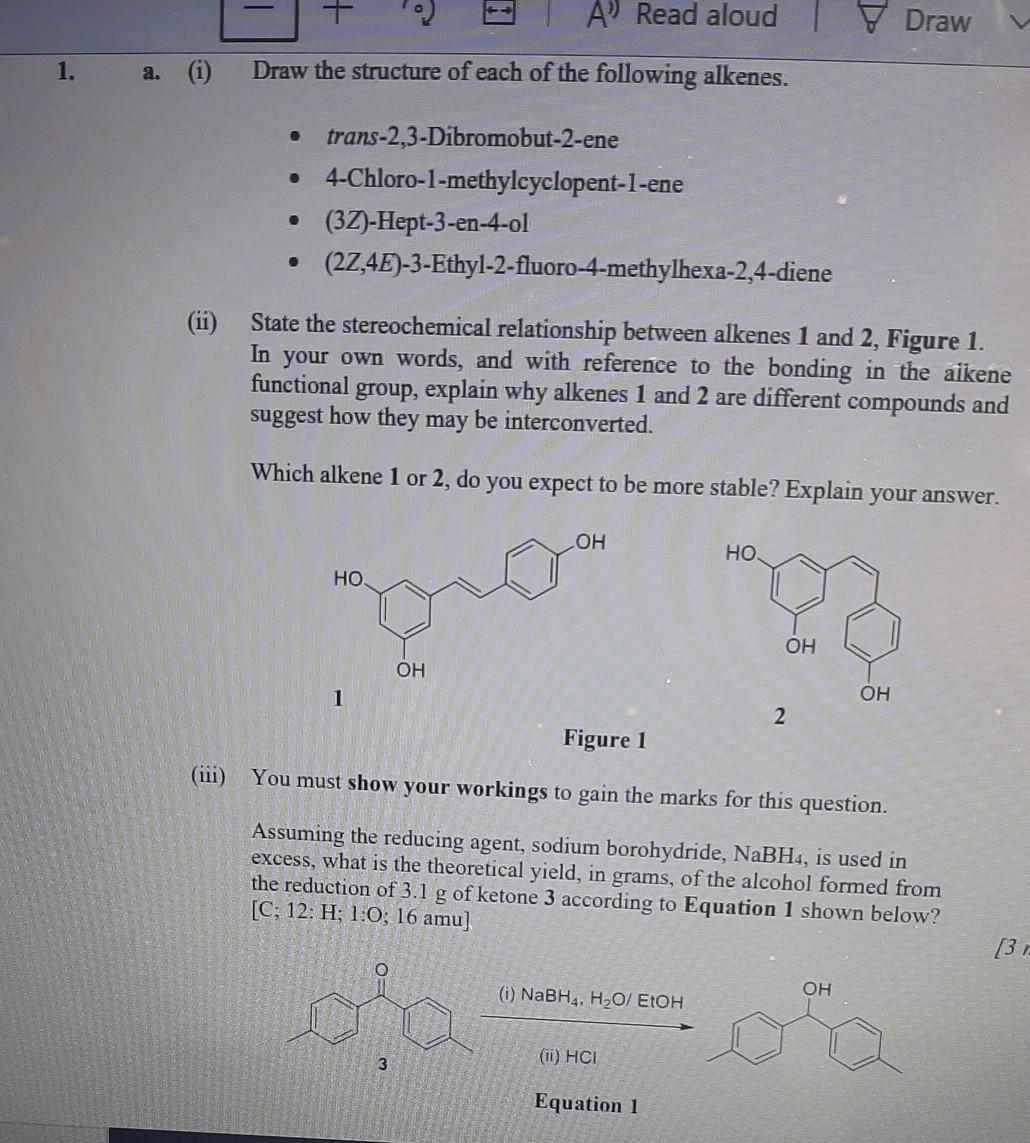
A' Read aloud | Draw 1. a. (i) Draw the structure of each of the following alkenes. . trans-2,3-Dibromobut-2-ene 4-Chloro-1-methylcyclopent-1-ene (3Z)-Hept-3-en-4-ol . (22,4E)-3-Ethyl-2-fluoro-4-methylhexa-2,4-diene (11) State the stereochemical relationship between alkenes 1 and 2, Figure 1. In your own words, and with reference to the bonding in the aikene functional group, explain why alkenes 1 and 2 are different compounds and suggest how they may be interconverted. Which alkene 1 or 2, do you expect to be more stable? Explain your answer. OH . . OH OH 1 OH 2 Figure 1 You must show your workings to gain the marks for this question. Assuming the reducing agent, sodium borohydride, NaBH4, is used in excess, what is the theoretical yield, in grams, of the alcohol formed from the reduction of 3.1 g of ketone 3 according to Equation 1 shown below? [C; 12: H; 1,0; 16 amu] 131 (i) NaBH4, H2O/ EtOH OH 3 (1) HCI Equation 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


