Question: A reversible, nonflow, constant volume process de- creases the internal energy by 316.5 kJ for 2.268 kg of a gas for which R=430 J/kg.K
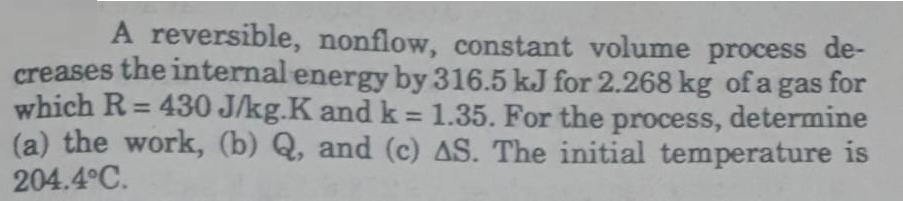
A reversible, nonflow, constant volume process de- creases the internal energy by 316.5 kJ for 2.268 kg of a gas for which R=430 J/kg.K and k = 1.35. For the process, determine (a) the work, (b) Q, and (c) AS. The initial temperature is 204.4C.
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Constant Volume K 2 a work por 6 from foot lawe first W par ok ... View full answer

Get step-by-step solutions from verified subject matter experts


