Question: A rigid tank whose volume is unknown is divided into two compartments by a partition. One side of the tank contains an ideal gas at
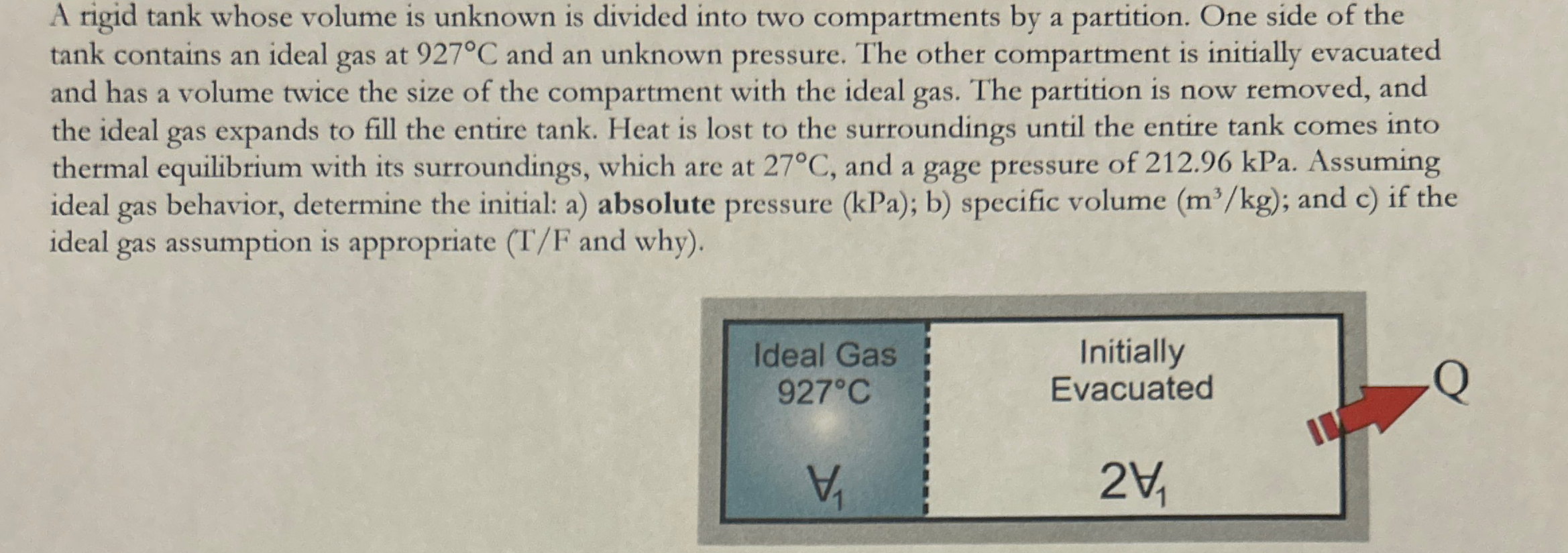
A rigid tank whose volume is unknown is divided into two compartments by a partition. One side of the
tank contains an ideal gas at and an unknown pressure. The other compartment is initially evacuated
and has a volume twice the size of the compartment with the ideal gas. The partition is now removed, and
the ideal gas expands to fill the entire tank. Heat is lost to the surroundings until the entire tank comes into
thermal equilibrium with its surroundings, which are at and a gage pressure of kPa Assuming
ideal gas behavior, determine the initial: a absolute pressure kPA; b specific volume mkg; and c if the ideal gas assumption is appropriate TF and why
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


