Question: = A sample of groundwater has percolated through a layer of gypsum (CaSO4) with Ksp = 4.9x10-5 = 104.3) is found to be 8.4x10-6 M
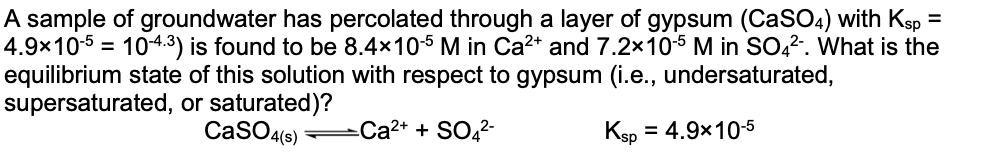
= A sample of groundwater has percolated through a layer of gypsum (CaSO4) with Ksp = 4.9x10-5 = 104.3) is found to be 8.4x10-6 M in Ca2+ and 7.2x10-5 M in SO42-. What is the equilibrium state of this solution with respect to gypsum (i.e., undersaturated, supersaturated, or saturated)? Ca2+ + SO42- Ksp = 4.9x10-5 CaSO4(s) =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


