Question: A sample of ( mathrm{N}_{2} mathrm{O}_{5} ) was placed in a container to allow the following reaction to occur: [ 2 mathrm{~N}_{2} mathrm{O}_{5}(g) ightarrow 4
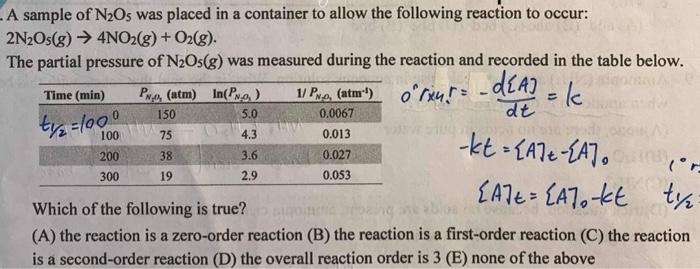
A sample of N2O5 was placed in a container to allow the following reaction to occur: 2N2O5(g)4NO2(g)+O2(g). The partial pressure of N2O5(g) was measured during the reaction and recorded in the table below. or4!=dtd[A]=kkt=[A]t[A]0[A]t=[A]0kt Which of the following is true? (A) the reaction is a zero-order reaction (B) the reaction is a first-order reaction (C) the reaction is a second-order reaction (D) the overall reaction order is 3(E) none of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


