Question: A scientist discovered a new element X that has two naturally occurring isotopes. The first isotope has a mass (ml) = 42.63 amu and abundance
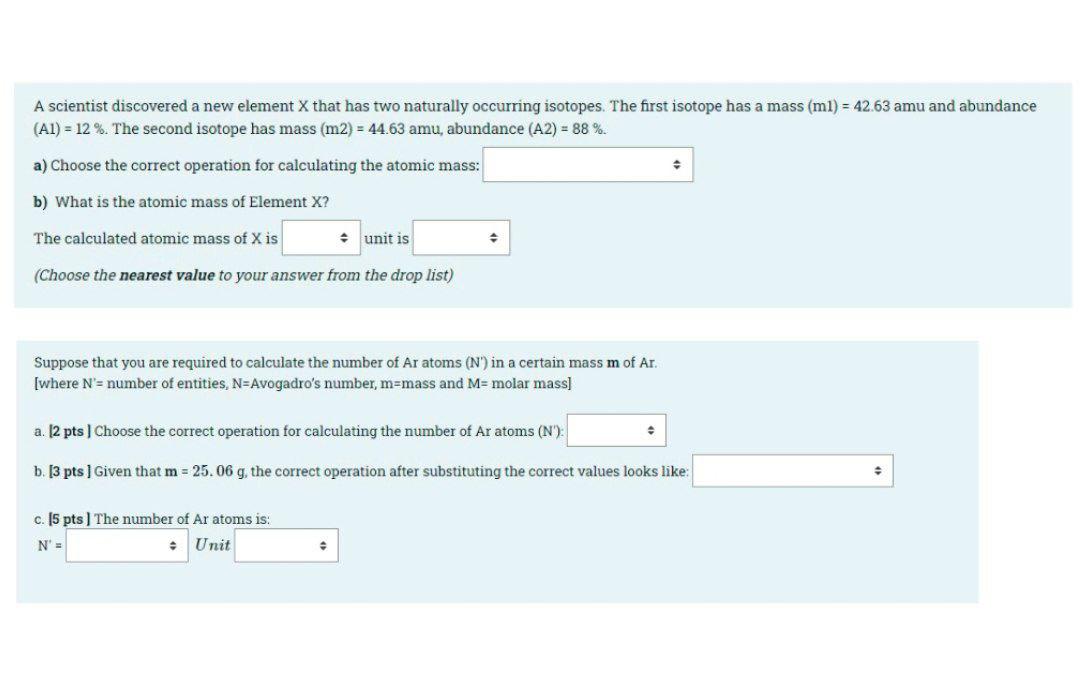
A scientist discovered a new element X that has two naturally occurring isotopes. The first isotope has a mass (ml) = 42.63 amu and abundance (Al) = 12%. The second isotope has mass (m2) = 44.63 amu, abundance (A2) = 88 %. a) Choose the correct operation for calculating the atomic mass: b) What is the atomic mass of Element X? The calculated atomic mass of X is unit is (Choose the nearest value to your answer from the drop list) Suppose that you are required to calculate the number of Ar atoms (N') in a certain mass m of Ar. [where N'= number of entities, N=Avogadro's number, m=mass and M= molar mass] a. 12 pts Choose the correct operation for calculating the number of Ar atoms (N'). b. [3 pts ] Given that m = 25.06 g, the correct operation after substituting the correct values looks like: c. 15 pts) The number of Ar atoms is: N' Unit
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


