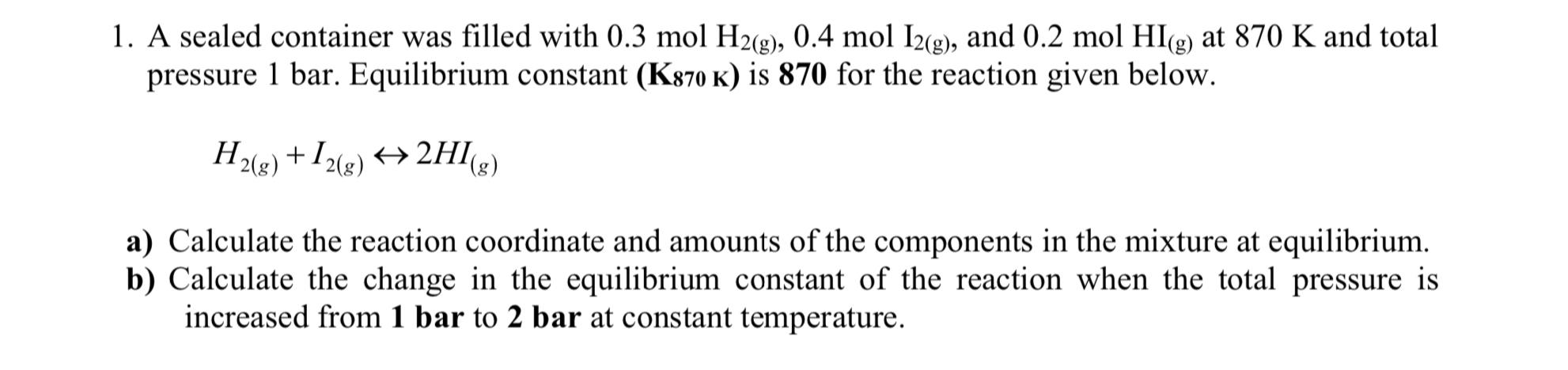
Question: A sealed container was filled with 0 . 3 m o l H 2 ( g ) , 0 . 4 m o l I
A sealed container was filled with and at and total pressure bar. Equilibrium constant is for the reaction given below.
a Calculate the reaction coordinate and amounts of the components in the mixture at equilibrium.
b Calculate the change in the equilibrium constant of the reaction when the total pressure is increased from bar to bar at constant temperature.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


