Question: 3. Consider the following reaction and kinetic data: 2 HgCl2(aq) + C2O4 (aq) 2 Cl-(aq) + Hg2Cl2(aq) + 2CO2(g) Expt. # [HgC](M) [CO2] (M)
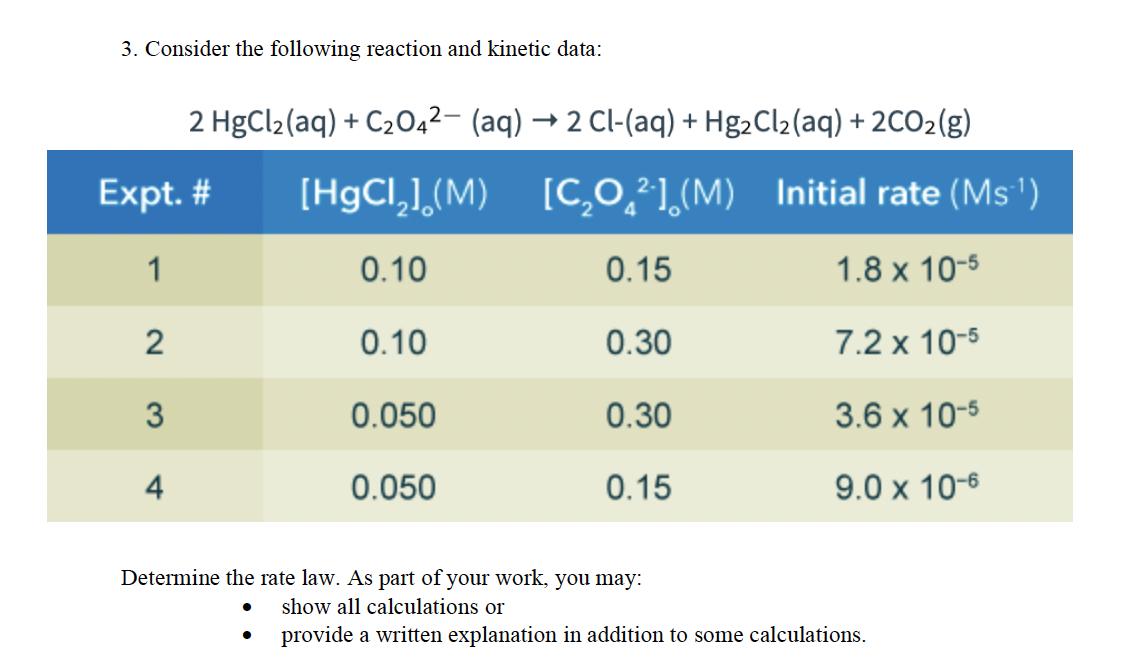
3. Consider the following reaction and kinetic data: 2 HgCl2(aq) + C2O4 (aq) 2 Cl-(aq) + Hg2Cl2(aq) + 2CO2(g) Expt. # [HgC](M) [CO2] (M) Initial rate (Ms) 1 0.10 0.15 1.8 x 10-5 2 0.10 0.30 7.2 x 10-5 3 0.050 0.30 3.6 x 10-5 4 0.050 0.15 9.0 x 10-6 Determine the rate law. As part of your work, you may: show all calculations or provide a written explanation in addition to some calculations.
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
To Solution For the given reaction rate law written as x Rate k Mgc1 60y 18x... View full answer

Get step-by-step solutions from verified subject matter experts


