Question: A sequential first-order reaction is: AkAIkIP where A decay to an intermediate I that subsequently decays to the product P. The rate law of the
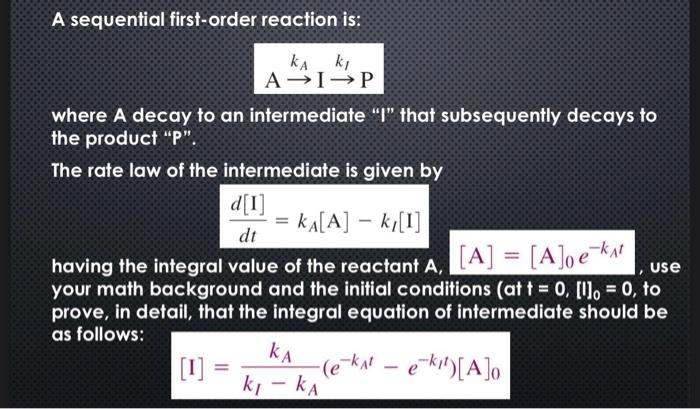
A sequential first-order reaction is: AkAIkIP where A decay to an intermediate "I" that subsequently decays to the product "P". The rate law of the intermediate is given by dtd[I]=kA[A]kI[I] having the integral value of the reactant A,[A]=[A]0ekAt, use your math background and the initial conditions (at t=0, []0=0, to prove, in detail, that the integral equation of intermediate should be as follows: [I]=kIkAkA(ekAteklt)[A]0
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


