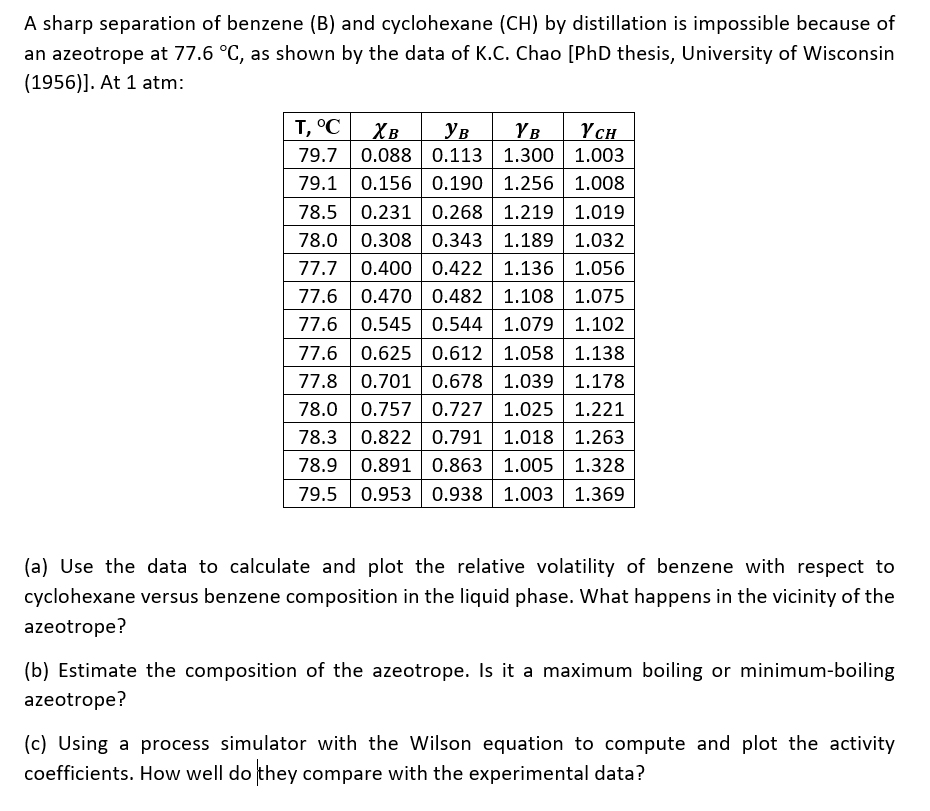
Question: A sharp separation of benzene ( B ) and cyclohexane ( C H ) by distillation is impossible because of an azeotrope at 7 7
A sharp separation of benzene and cyclohexane by distillation is impossible because of
an azeotrope at as shown by the data of KC Chao PhD thesis, University of Wisconsin
At atm:
a Use the data to calculate and plot the relative volatility of benzene with respect to
cyclohexane versus benzene composition in the liquid phase. What happens in the vicinity of the
azeotrope?
b Estimate the composition of the azeotrope. Is it a maximum boiling or minimumboiling
azeotrope?
c Using a process simulator with the Wilson equation to compute and plot the activity
coefficients. How well do they compare with the experimental data?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


