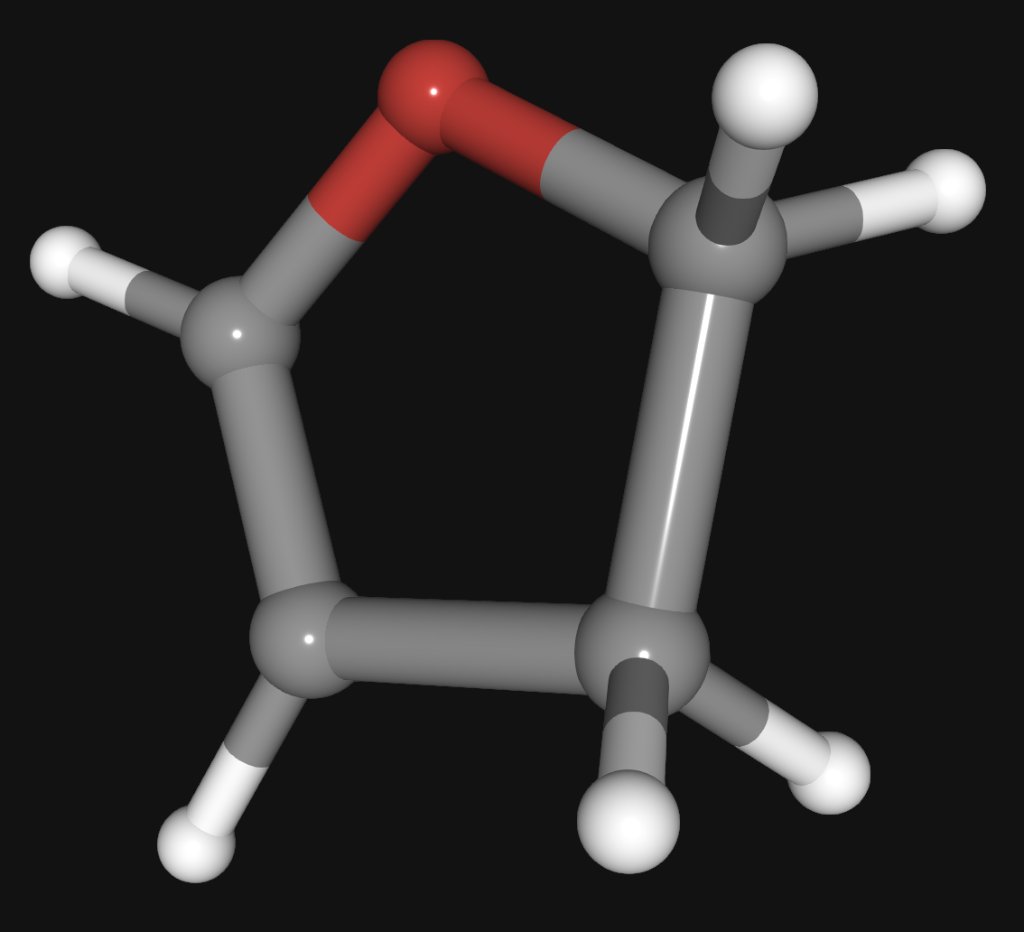
Question: A skeletal structure for molecule C (C 4 H 6 O), which shows only how the atoms are linked without lone pairs or pi electrons,
A skeletal structure for molecule C (C4H6O), which shows only how the atoms are linked without lone pairs or pi electrons, is below. Carbons are shown in gray, oxygens in red, and hydrogens in white. Enter the number of double bonds and lone pairs in the best Lewis structure of C.
Four skeletal structures lacking lone pairs and pi bonds are shown below. For which of the structures is it not possible to draw a Lewis structure lacking formal charges while satisfying the octet rule? Carbons are gray, hydrogens are white, oxygens are red, and nitrogens are blue.
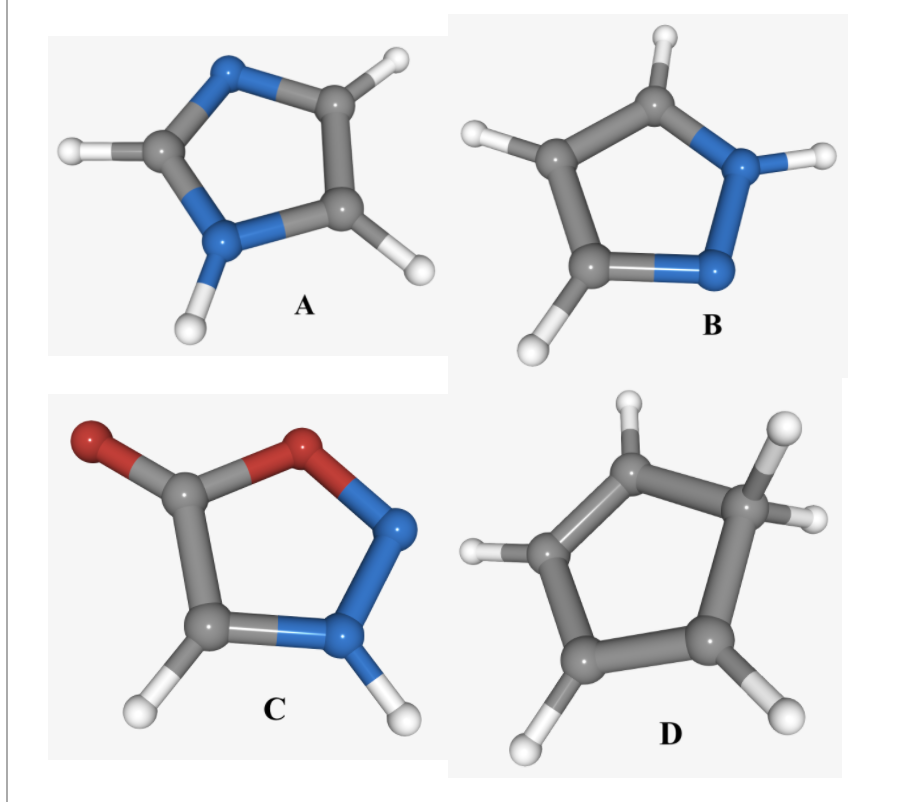
B D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


