Question: A solid mixture consists of 4 5 . 9 g of K N O 3 ( potassium nitrate ) and 8 . 1 g of
A solid mixture consists of of potassium nitrate and of potassium sulfate The mixture is added to of water.
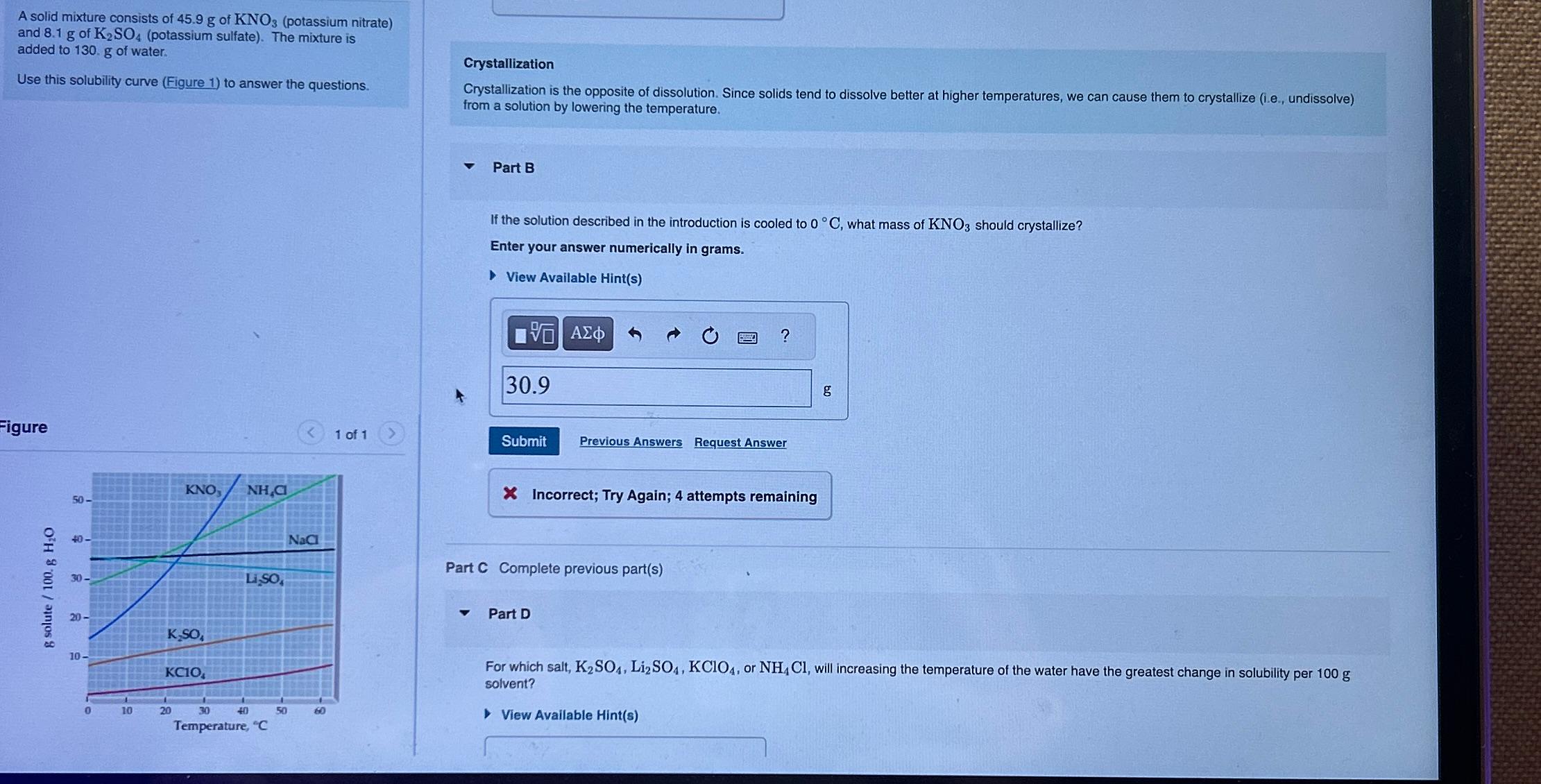
Use this solubility curve Figure to answer the questions.
Figure
of
of
Part C Complete previous parts
Part D
For which salt, or will increasing the temperature of the water have the greatest change in solubility per solvent?
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


