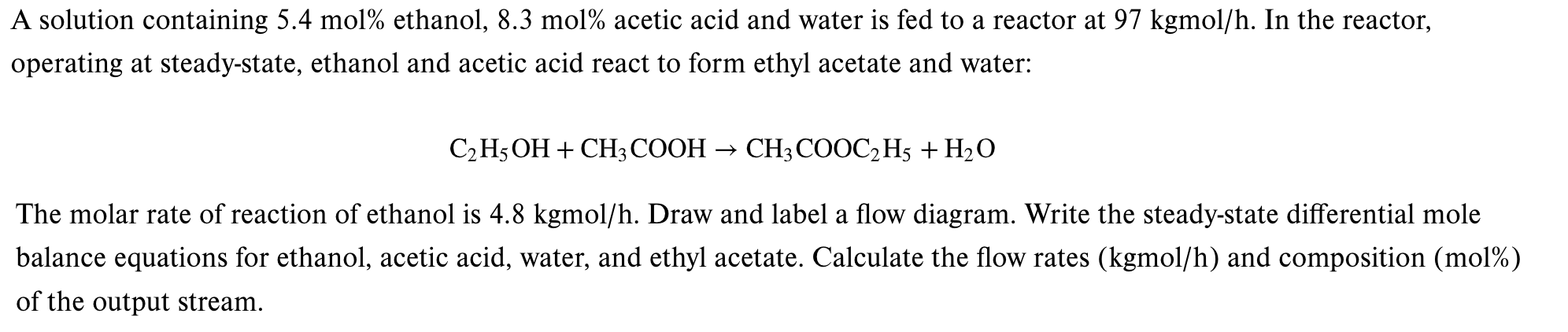
Question: A solution containing 5 . 4 mol % ethanol, 8 . 3 mol % acetic acid and water is fed to a reactor at 9
A solution containing mol ethanol, mol acetic acid and water is fed to a reactor at kgmo In the reactor,
operating at steadystate, ethanol and acetic acid react to form ethyl acetate and water:
The molar rate of reaction of ethanol is kgmo Draw and label a flow diagram. Write the steadystate differential mole
balance equations for ethanol, acetic acid, water, and ethyl acetate. Calculate the flow rates kgmo and composition mol
of the output stream.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


