Question: A solution is made by dissolving 0.610mol of nonelectrolyte solute in 867g of benzene. Calculate the freezing point, Tf, and boiling point, Tb, of the
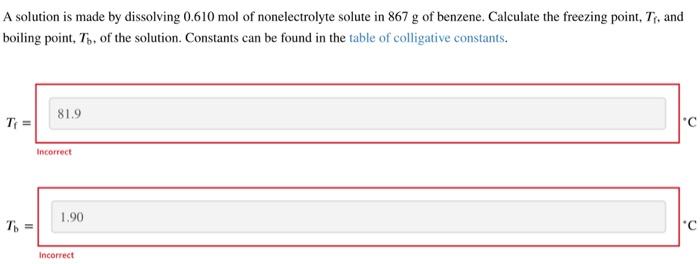
A solution is made by dissolving 0.610mol of nonelectrolyte solute in 867g of benzene. Calculate the freezing point, Tf, and boiling point, Tb, of the solution. Constants can be found in the table of colligative constants
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


