Question: A solution is made by dissolving a small amount of KCl into a large container of water at 20.0C. After mixing at constant pressure the
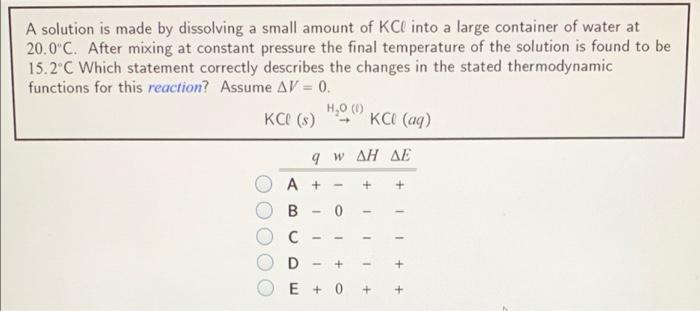
A solution is made by dissolving a small amount of KCl into a large container of water at 20.0C. After mixing at constant pressure the final temperature of the solution is found to be 15.2C Which statement correctly describes the changes in the stated thermodynamic functions for this reaction? Assume AV = 0. H,0 ( KCL (3) KCI (aq) 9 WAH AE A+ B 0 1 1 1 - - C D + + 1 E + 0 + +
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


