Question: A solution is made using 19.3 percent by mass CH2Cl2 in CHCl3. At 30C, the vapor pressure of pure CH2Cl2 is 490 mmHg, and the
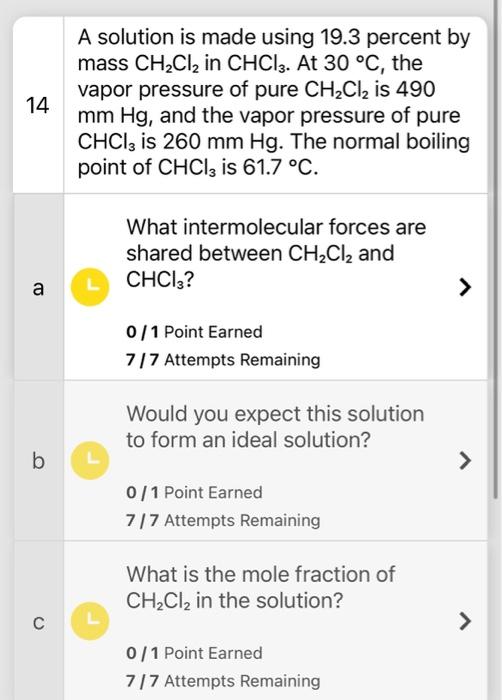
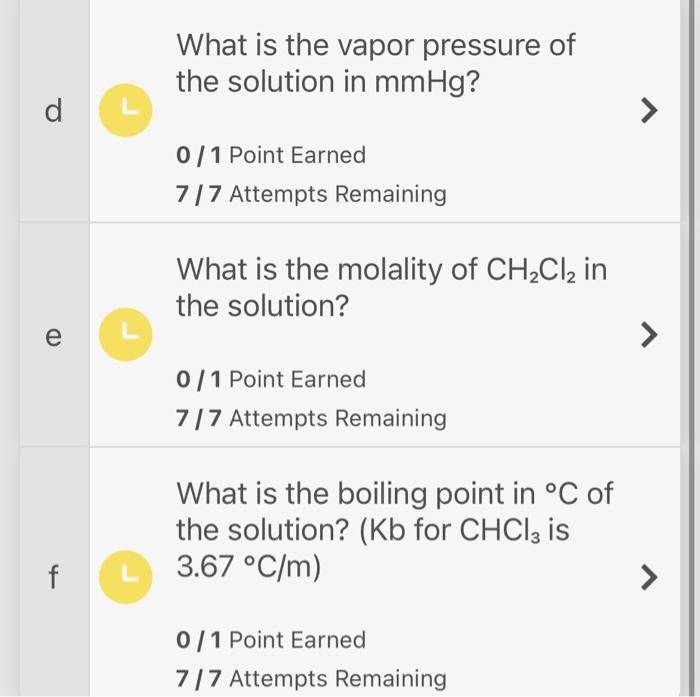
A solution is made using 19.3 percent by mass CH2Cl2 in CHCl3. At 30C, the vapor pressure of pure CH2Cl2 is 490 mmHg, and the vapor pressure of pure CHCl3 is 260mmHg. The normal boiling point of CHCl3 is 61.7C. What intermolecular forces are shared between CH2Cl2 and CHCl3 ? 0 / 1 Point Earned 7/7 Attempts Remaining Would you expect this solution to form an ideal solution? 0/1 Point Earned 7 / 7 Attempts Remaining What is the mole fraction of CH2Cl2 in the solution? 0/1 Point Earned 7/7 Attempts Remaining What is the vapor pressure of the solution in mmHg ? 0/1 Point Earned 7/7 Attempts Remaining What is the molality of CH2Cl2 in the solution? 0/1 Point Earned 7/7 Attempts Remaining What is the boiling point in C of the solution? ( Kb for CHCl3 is 3.67C/m) 0/ 1 Point Earned 7/7 Attempts Remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


