Question: A solution is prepared at 25C that is initially 0.22M in trimethylamine ((CH3)3N), a weak base with Kb=7.4104, and 0.27M in trimethylammonium chloride ((CH3)3NHCl). Calculate
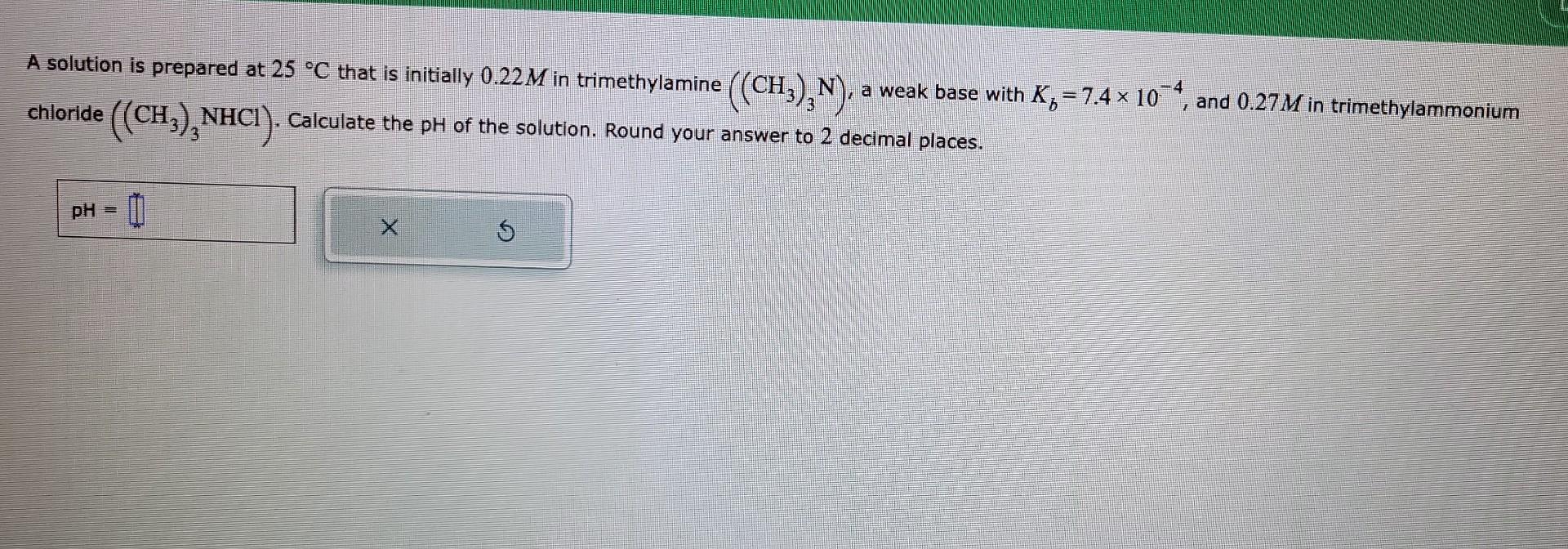
A solution is prepared at 25C that is initially 0.22M in trimethylamine ((CH3)3N), a weak base with Kb=7.4104, and 0.27M in trimethylammonium chloride ((CH3)3NHCl). Calculate the pH of the solution. Round your answer to 2 decimal places
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


