Question: A solution is prepared by adding 49 mg of sodium acetate (CH3COONa) to 1 L of distilled water. The acetate ion (CH3COO-) is the conjugate
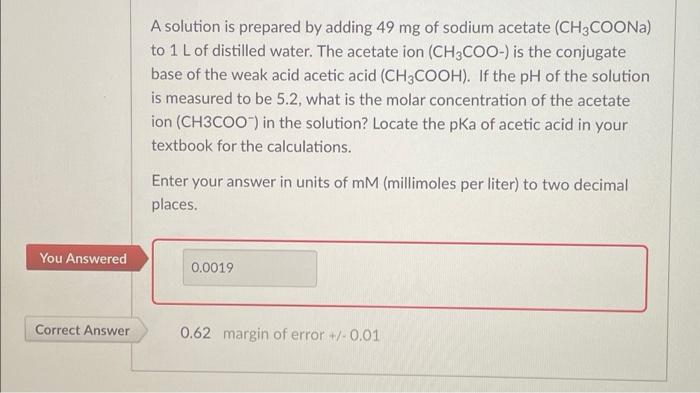
A solution is prepared by adding 49 mg of sodium acetate (CH3COONa) to 1 L of distilled water. The acetate ion (CH3COO-) is the conjugate base of the weak acid acetic acid (CH3COOH). If the pH of the solution is measured to be 5.2, what is the molar concentration of the acetate ion (CH3COO-) in the solution? Locate the pKa of acetic acid in your textbook for the calculations. Enter your answer in units of mm (millimoles per liter) to two decimal places. You Answered 0.0019 Correct Answer 0.62 margin of error +/-0.01
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


