Question: A solution is prepared that is initially 0.088M in nitrous acid (HNO,) and 0.34 M in potassium nitrite (KNO,) Complete the reaction table below, so
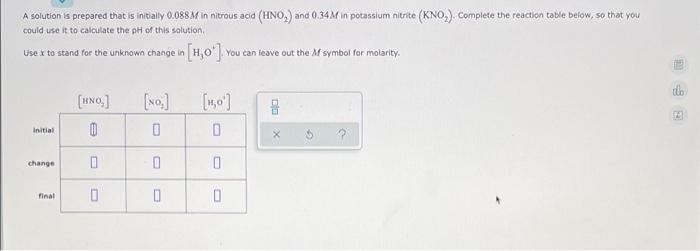
A solution is prepared that is initially 0.088M in nitrous acid (HNO,) and 0.34 M in potassium nitrite (KNO,) Complete the reaction table below, so that you could use it to calculate the pH of this solution Use x to stand for the unknown change in (1,0'] You can leave out the M symbol for molarity til [no] [no] 0 [1,0) 0 DO Initial 0 X 5 change 0 0 Final 0 0 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


