Question: A solution is prepared that is initially 0.44M in pyridine (C5H5N), a weak base, and 0.41M in pyridinium bromide (C5H5NHBr), Complete the reaction table below,
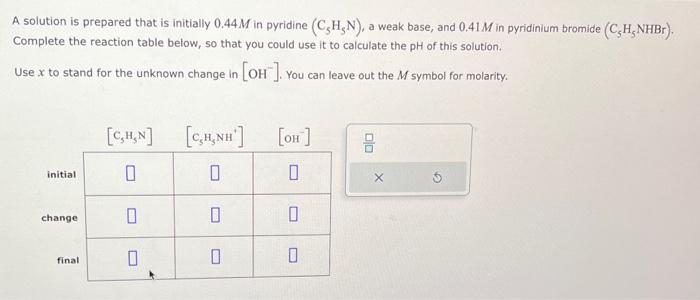
A solution is prepared that is initially 0.44M in pyridine (C5H5N), a weak base, and 0.41M in pyridinium bromide (C5H5NHBr), Complete the reaction table below, so that you could use it to calculate the pH of this solution. Use x to stand for the unknown change in [OH]. You can leave out the M symbol for molarity
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


