Question: A steady - state distillation column with a partial reboiler and a total condenser is employed for acetone - ethanol separation. There are two feeds
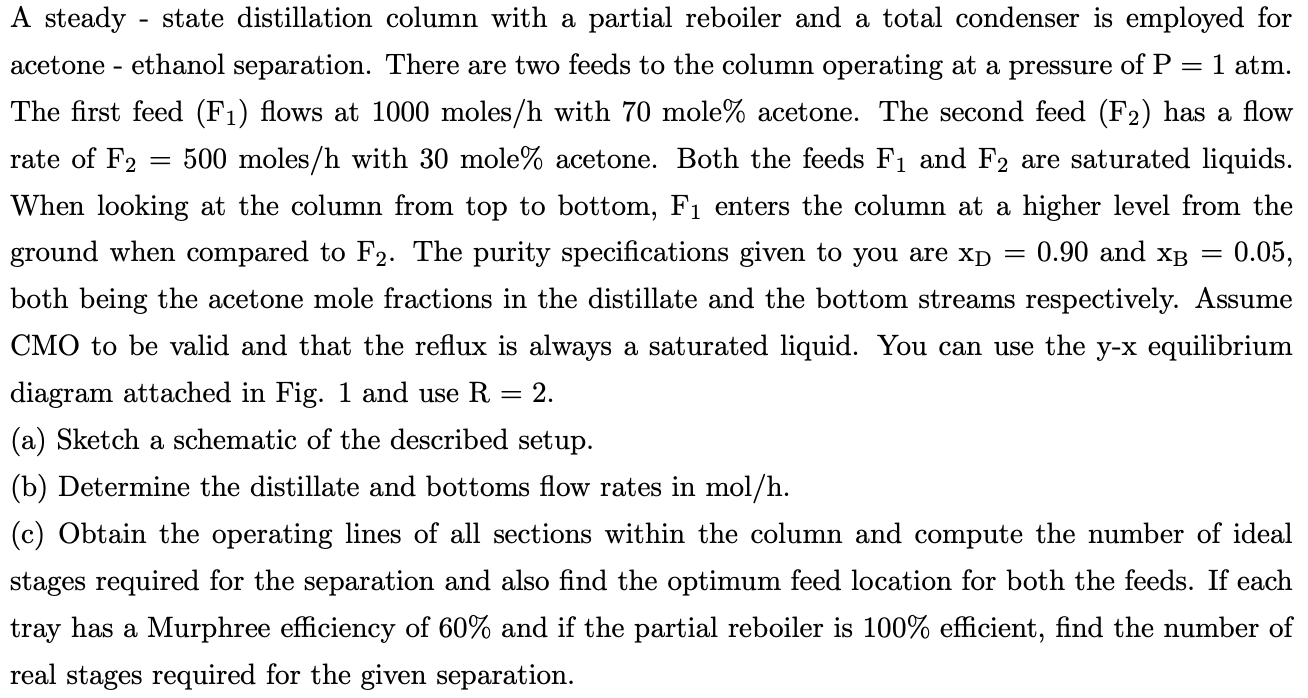
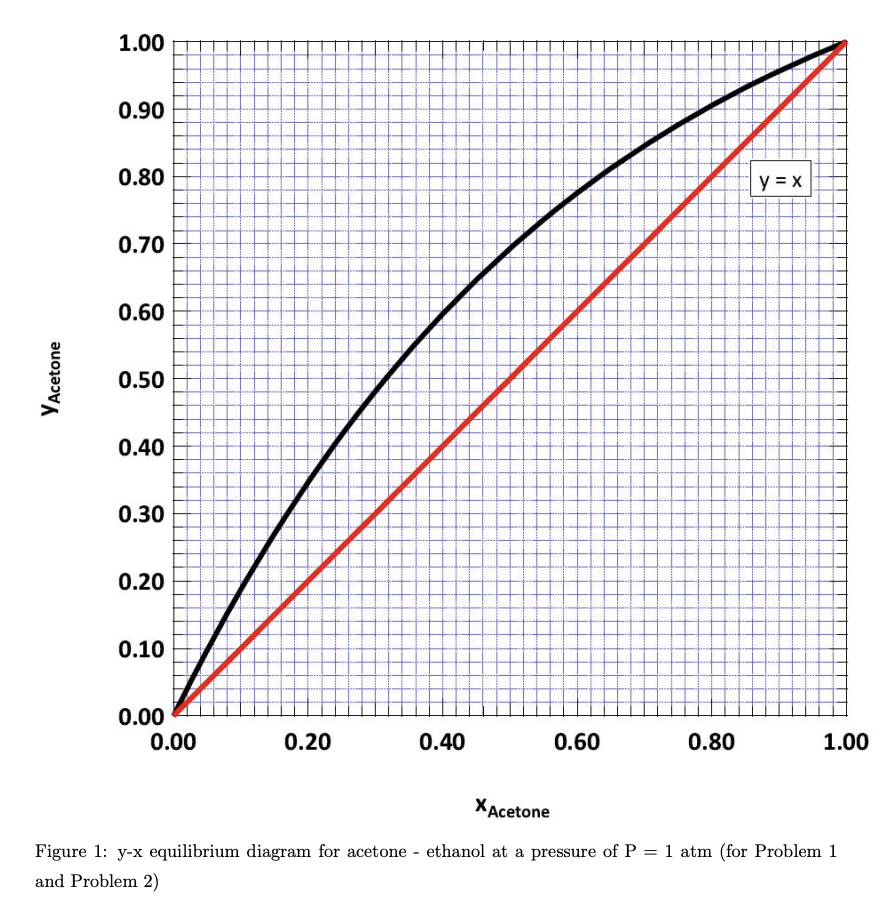
A steady - state distillation column with a partial reboiler and a total condenser is employed for acetone - ethanol separation. There are two feeds to the column operating at a pressure of P = 1 atm. The first feed (F1) flows at 1000 moles/h with 70 mole% acetone. The second feed (F2) has a flow rate of F2 500 moles/h with 30 mole% acetone. Both the feeds F1 and F2 are saturated liquids. When looking at the column from top to bottom, F enters the column at a higher level from the ground when compared to F2. The purity specifications given to you are xp = 0.90 and XB = = 0.05, both being the acetone mole fractions in the distillate and the bottom streams respectively. Assume CMO to be valid and that the reflux is always a saturated liquid. You can use the y-x equilibrium diagram attached in Fig. 1 and use R = = 2. (a) Sketch a schematic of the described setup. (b) Determine the distillate and bottoms flow rates in mol/h. (c) Obtain the operating lines of all sections within the column and compute the number of ideal stages required for the separation and also find the optimum feed location for both the feeds. If each tray has a Murphree efficiency of 60% and if the partial reboiler is 100% efficient, find the number of real stages required for the given separation. 1.00 0.90 0.80 y = X 0.70 0.60 Y Acetone 0.50 0.40 0.30 0.20 0.10 0.00 0.00 0.20 0.40 0.60 0.80 1.00 X Acetone ethanol at a pressure of P = 1 atm (for Problem 1 = Figure 1: y-x equilibrium diagram for acetone and Problem 2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


