Question: .Hckel MO Theory assumes that the overlap integral Hij between p atomic orbitals (pAOs) that do not belong to neighboring Carbon atoms is zero.
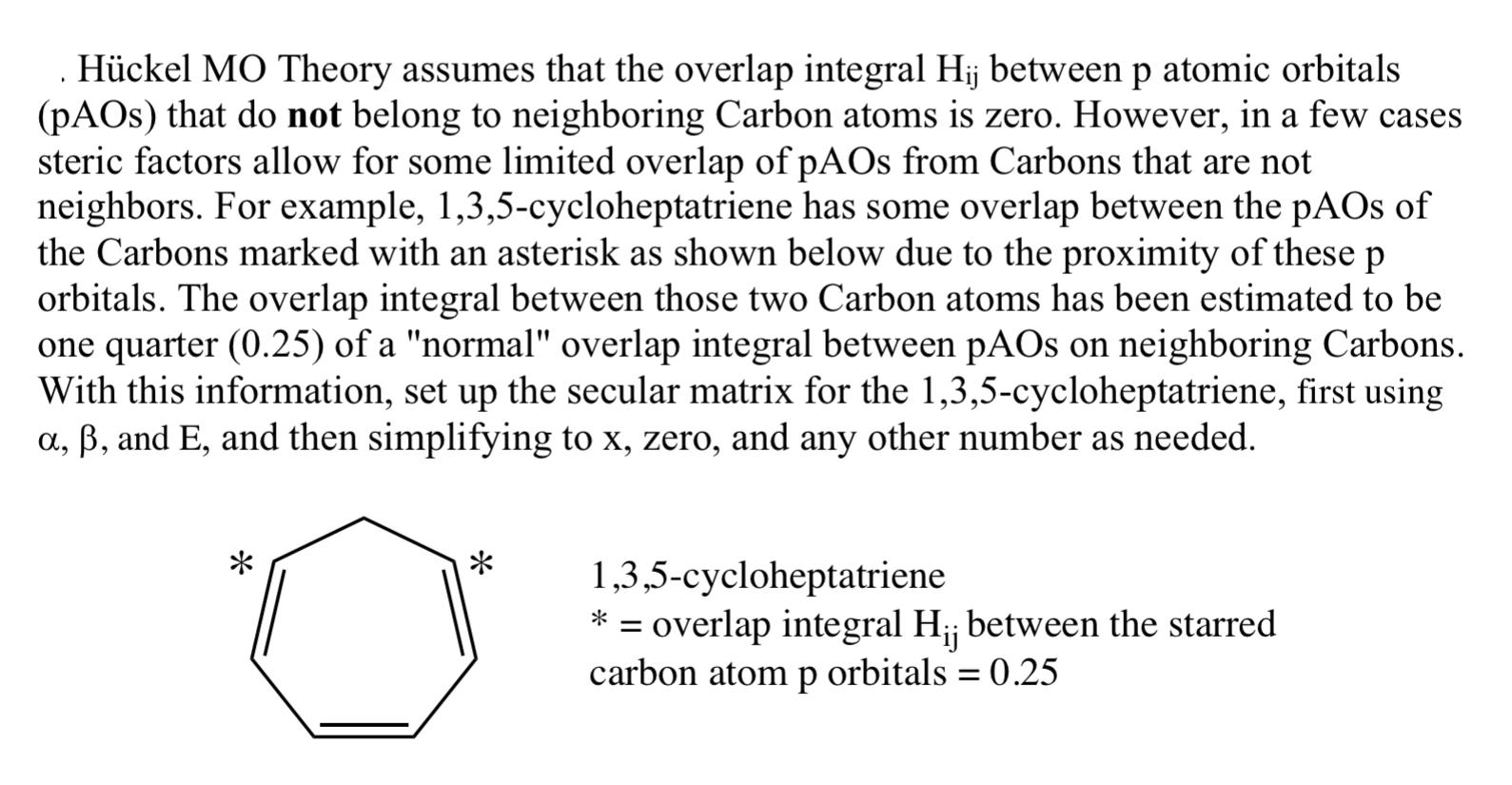
.Hckel MO Theory assumes that the overlap integral Hij between p atomic orbitals (pAOs) that do not belong to neighboring Carbon atoms is zero. However, in a few cases steric factors allow for some limited overlap of pAOs from Carbons that are not neighbors. For example, 1,3,5-cycloheptatriene has some overlap between the pAOs of the Carbons marked with an asterisk as shown below due to the proximity of these p orbitals. The overlap integral between those two Carbon atoms has been estimated to be one quarter (0.25) of a "normal" overlap integral between pAOs on neighboring Carbons. With this information, set up the secular matrix for the 1,3,5-cycloheptatriene, first using a, , and E, and then simplifying to x, zero, and any other number as needed. * * 1,3,5-cycloheptatriene = overlap integral H; between the starred carbon atom p orbitals = 0.25
Step by Step Solution
There are 3 Steps involved in it
Here is the secular matri... View full answer

Get step-by-step solutions from verified subject matter experts


