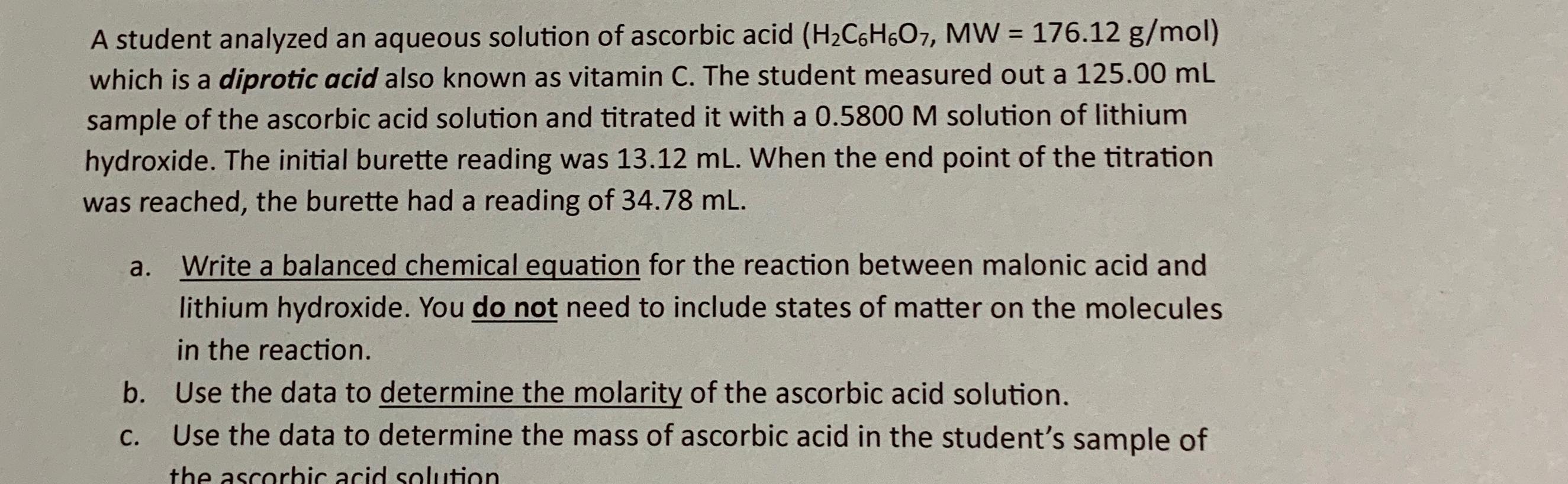
Question: A student analyzed an aqueous solution of ascorbic acid ) = ( 1 7 6 . 1 2 g m o l which is a
A student analyzed an aqueous solution of ascorbic acid which is a diprotic acid also known as vitamin C The student measured out a sample of the ascorbic acid solution and titrated it with a solution of lithium hydroxide. The initial burette reading was When the end point of the titration was reached, the burette had a reading of
a Write a balanced chemical equation for the reaction between malonic acid and lithium hydroxide. You do not need to include states of matter on the molecules in the reaction.
b Use the data to determine the molarity of the ascorbic acid solution.
c Use the data to determine the mass of ascorbic acid in the student's sample of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


