Question: A student plotted the concentration versus time data in lab, and came up with these three plots of [A] vs. time (R2=0.9882), In [A] vs.
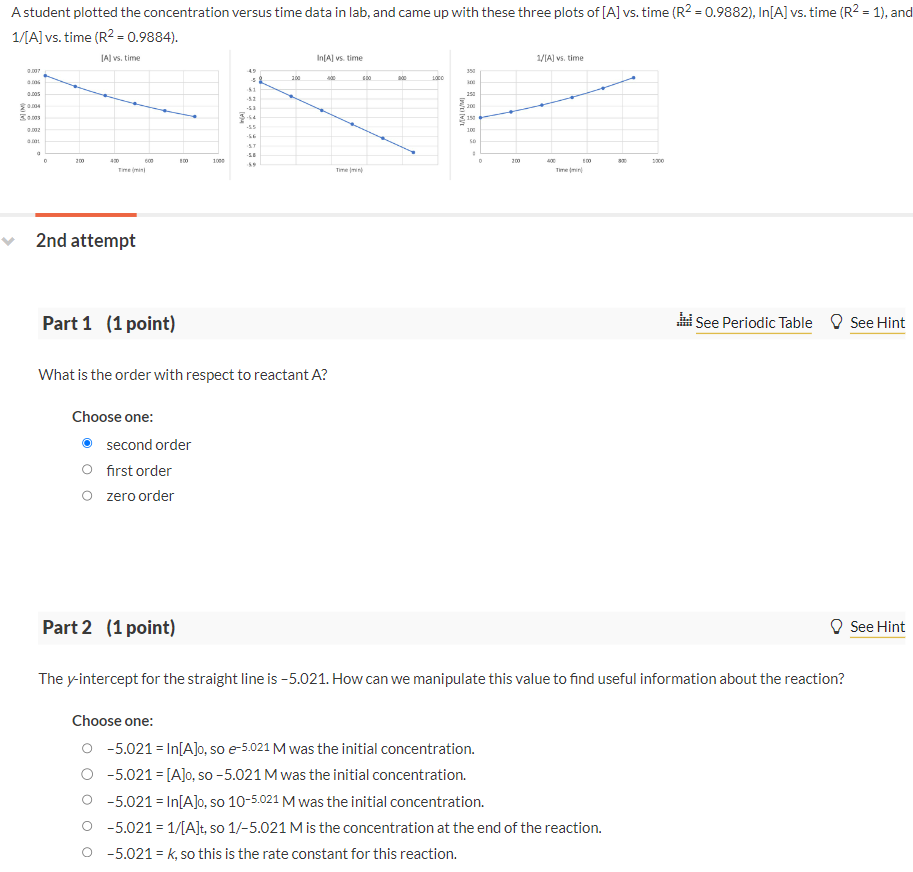
A student plotted the concentration versus time data in lab, and came up with these three plots of [A] vs. time (R2=0.9882), In [A] vs. time (R2=1), an 1/[A] vs. time (R2=0.9884). 2nd attempt Part 1 (1 point) see Periodic Table See Hint What is the order with respect to reactant A ? Choose one: second order first order zero order Part 2 (1 point) See Hint The y-intercept for the straight line is 5.021. How can we manipulate this value to find useful information about the reaction? Choose one: 5.021=ln[A]0, so e5.021M was the initial concentration. 5.021=[A]0, so 5.021M was the initial concentration. 5.021=ln[A]0, so 105.021M was the initial concentration. 5.021=1/[A]t, so 1/5.021M is the concentration at the end of the reaction. 5.021=k, so this is the rate constant for this reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


