Question: A student prepared benzil according to the experimental procedure using 2.58g. of benzoin and 12mL of nitric acid. The mass of the crude benzil product
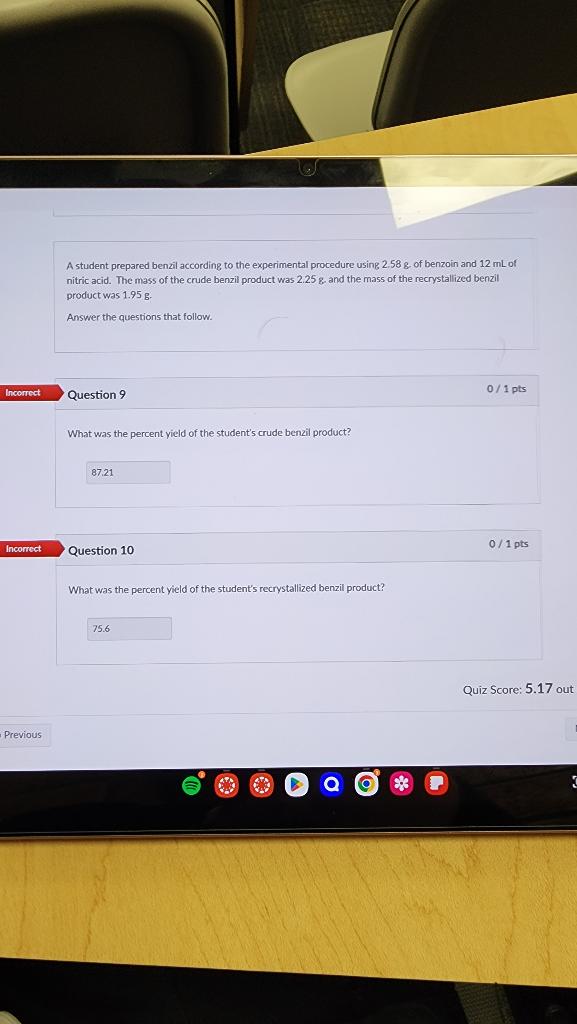
A student prepared benzil according to the experimental procedure using 2.58g. of benzoin and 12mL of nitric acid. The mass of the crude benzil product was 2.25g. and the mass of the recrystallized benzil product was 1.95g. Answer the questions that follow. Question 9 0/1pts What was the percent yield of the student's crude benzil product? Question 10 What was the percent yield of the student's recrystallized benzil product
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


