Question: A system combining a solid-oxide fuel cell with a gas turbine has been proved to achieve higher operating efficiencies at high pressures. The exhaust gases
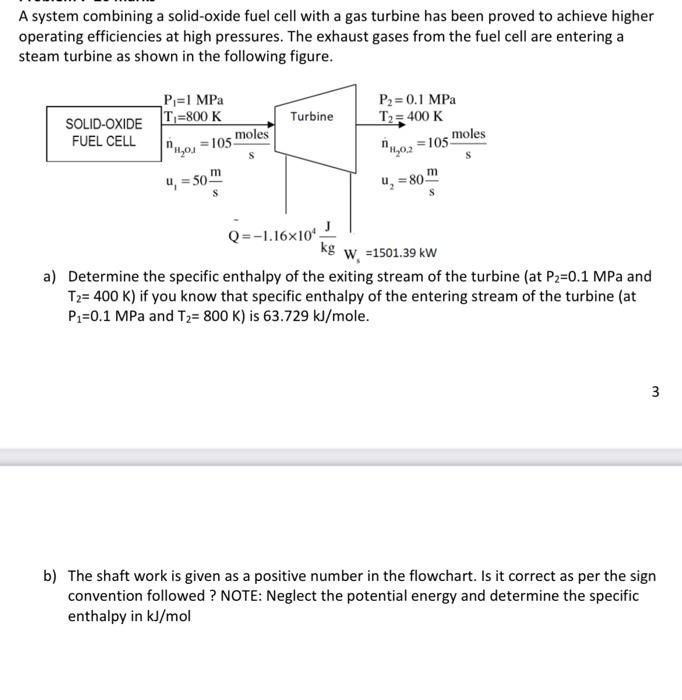
A system combining a solid-oxide fuel cell with a gas turbine has been proved to achieve higher operating efficiencies at high pressures. The exhaust gases from the fuel cell are entering a steam turbine as shown in the following figure. a) Determine the specific enthalpy of the exiting stream of the turbine (at P2=0.1MPa and T2=400K ) if you know that specific enthalpy of the entering stream of the turbine (at P1=0.1MPa and T2=800K ) is 63.729kJ/mole. 3 b) The shaft work is given as a positive number in the flowchart. Is it correct as per the sign convention followed? NOTE: Neglect the potential energy and determine the specific enthalpy in kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


