Question: (a) The benzylamide is the product obtained from the liquidphase reaction of ammonia and benzoyl chloride. The production capacity of benzylamide per year is 4
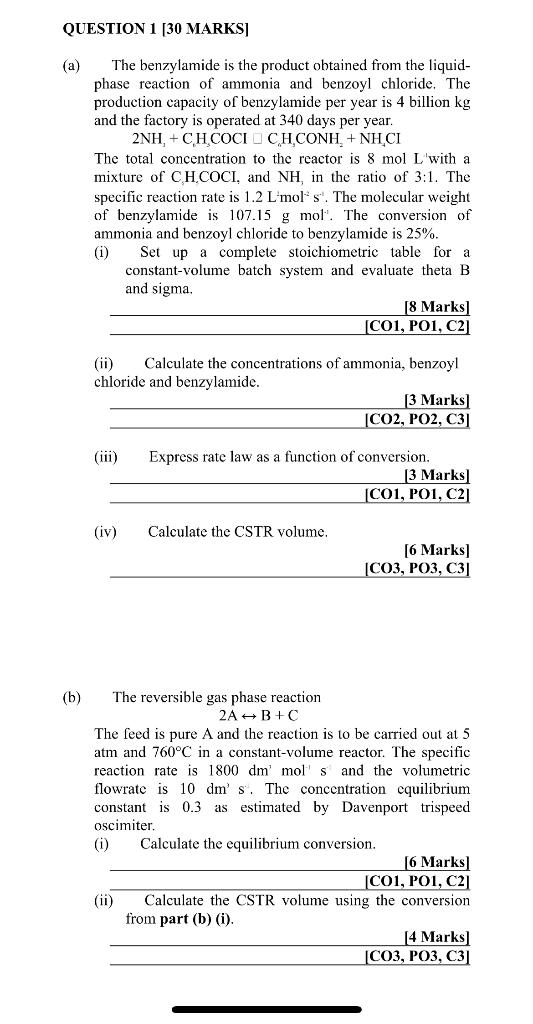
(a) The benzylamide is the product obtained from the liquidphase reaction of ammonia and benzoyl chloride. The production capacity of benzylamide per year is 4 billion kg and the factory is operated at 340 days per year. 2NH3+C6H3COCIC6H3CONH2+NH4CI The total concentration to the reactor is 8molL with a mixture of CHHOCI, and NH3 in the ratio of 3:1. The specific reaction rate is 1.2Lmol2s1. The molecular weight of benzylamide is 107.15gmol1. The conversion of ammonia and benzoyl chloride to benzylamide is 25%. (i) Set up a complete stoichiometric table for a constant-volume batch system and evaluate theta B and sigma. [8 Marks] [CO1, PO1, C2] (ii) Calculate the concentrations of ammonia, benzoyl chloride and benzylamide. [3Marks][CO2,PO2,C3] (iii) Express rate law as a function of conversion. (iv) Calculate the CSTR volume. [6Marks][CO3,PO3,C3] (b) The reversible gas phase reaction 2AB+C The feed is pure A and the reaction is to be carried out at 5 atm and 760C in a constant-volume reactor. The specific reaction rate is 1800dmmol1s and the volumetric flowrate is 10dms. The concentration equilibrium constant is 0.3 as estimated by Davenport trispeed oscimiter. (i) Calculate the equilibrium conversion. [6 Marks] [CO1, PO1, C2] (ii) Calculate the CSTR volume using the conversion from part (b) (i)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


