Question: a. There are three diazines (C4H4N2) differing in the relative arrangements of the two nitrogens in the six-membered ring. i. Why is it difficult to
a. There are three diazines (C4H4N2) differing in the relative arrangements of the two nitrogens in the six-membered ring.
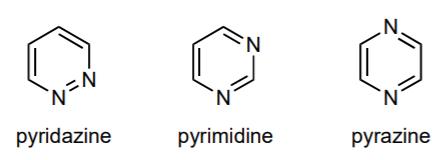
i. Why is it difficult to diprotonate a diazine? 1 mark
ii. Which is the only chlorodiazine that does not undergo easy nucleophilic substitution of the chloro group, and why? 2 mark
iii. The 1,3-diazine derivative, 4-amino-5-cyano-2-methylpyrimidine 6, is an intermediate in a synthesis of thiamine (vitamin B1). The synthesis of 6 is outlined in the following scheme.
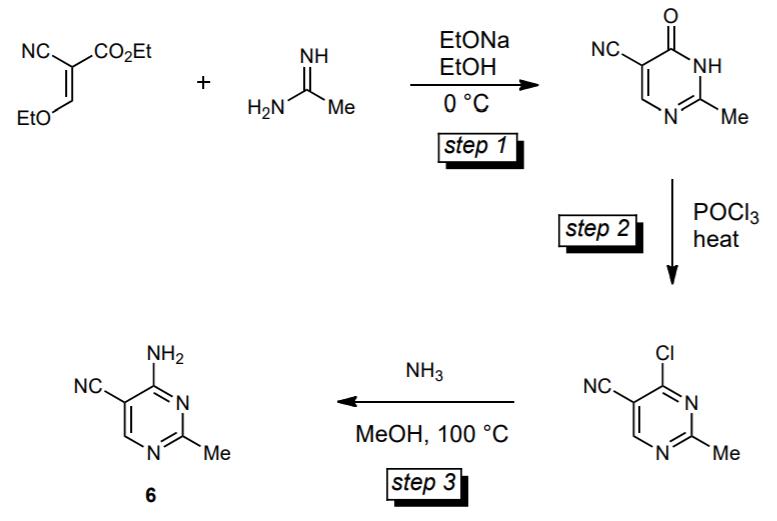
Outline a reasonable mechanism for step 1 of the sequence. 5 marks
.N. N=N pyridazine pyrimidine pyrazine
Step by Step Solution
3.51 Rating (144 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


