Question: A thyroid cancer patient ingests a single dose of radioactive iodine-131 to kill the cancer cells. Iodine-131 has an effective half-life of approximately 7.71
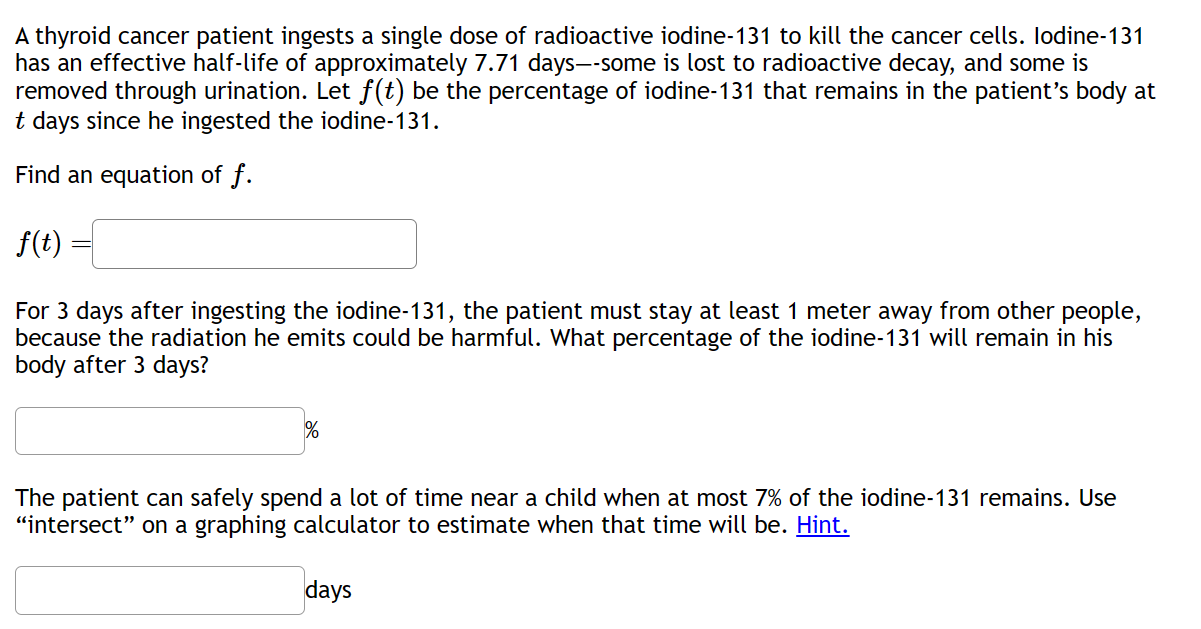
A thyroid cancer patient ingests a single dose of radioactive iodine-131 to kill the cancer cells. Iodine-131 has an effective half-life of approximately 7.71 days--some is lost to radioactive decay, and some is removed through urination. Let f(t) be the percentage of iodine-131 that remains in the patient's body at t days since he ingested the iodine-131. Find an equation of f. f(t) For 3 days after ingesting the iodine-131, the patient must stay at least 1 meter away from other people, because the radiation he emits could be harmful. What percentage of the iodine-131 will remain in his body after 3 days? The patient can safely spend a lot of time near a child when at most 7% of the iodine-131 remains. Use "intersect" on a graphing calculator to estimate when that time will be. Hint. days
Step by Step Solution
There are 3 Steps involved in it
Solutions we can also solve last question usin... View full answer

Get step-by-step solutions from verified subject matter experts


