Question: A. Using either HuLiS or a Frost circle, create an orbital energy diagram for the cyclopentadienyl anion. Provide the diagram in the box below, indicating
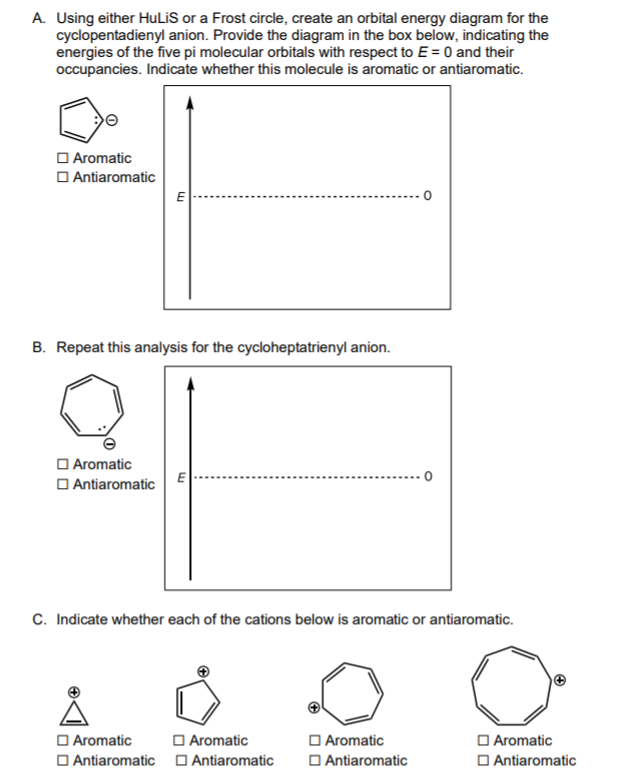
A. Using either HuLiS or a Frost circle, create an orbital energy diagram for the cyclopentadienyl anion. Provide the diagram in the box below, indicating the energies of the five pi molecular orbitals with respect to E = 0 and their occupancies. Indicate whether this molecule is aromatic or antiaromatic. Aromatic Antiaromatic E B. Repeat this analysis for the cycloheptatrienyl anion. Aromatic Antiaromatic E 0 C. Indicate whether each of the cations below is aromatic or antiaromatic. Aromatic Aromatic Antiaromatic Antiaromatic Aromatic Antiaromatic Aromatic Antiaromatic
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


