Question: a) Using the data provided in the table below, determine AG for the following reaction A+B C Compound AGF (kJ/mol) 387.0 B 519.0 491.0 AG:
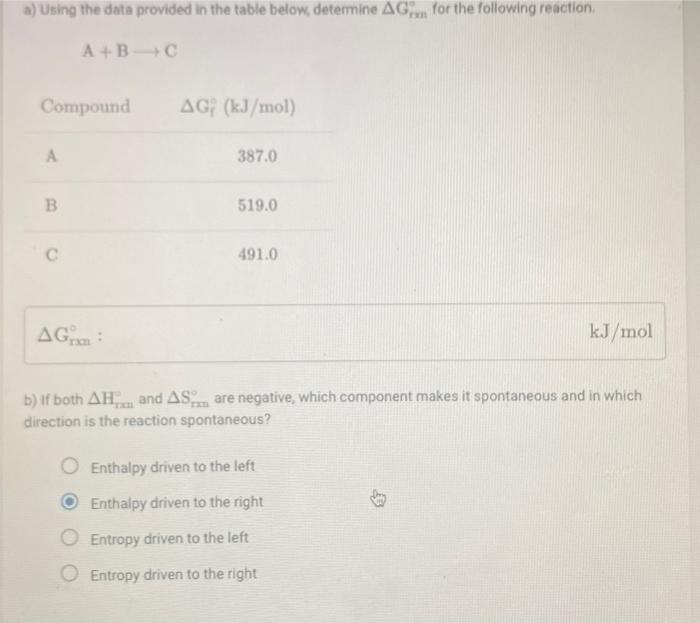
a) Using the data provided in the table below, determine AG for the following reaction A+B C Compound AGF (kJ/mol) 387.0 B 519.0 491.0 AG: kJ/mol b) if both AH... and AS...are negative, which component makes it spontaneous and in which direction is the reaction spontaneous? Enthalpy driven to the left Enthalpy driven to the right Entropy driven to the left Entropy driven to the right
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


