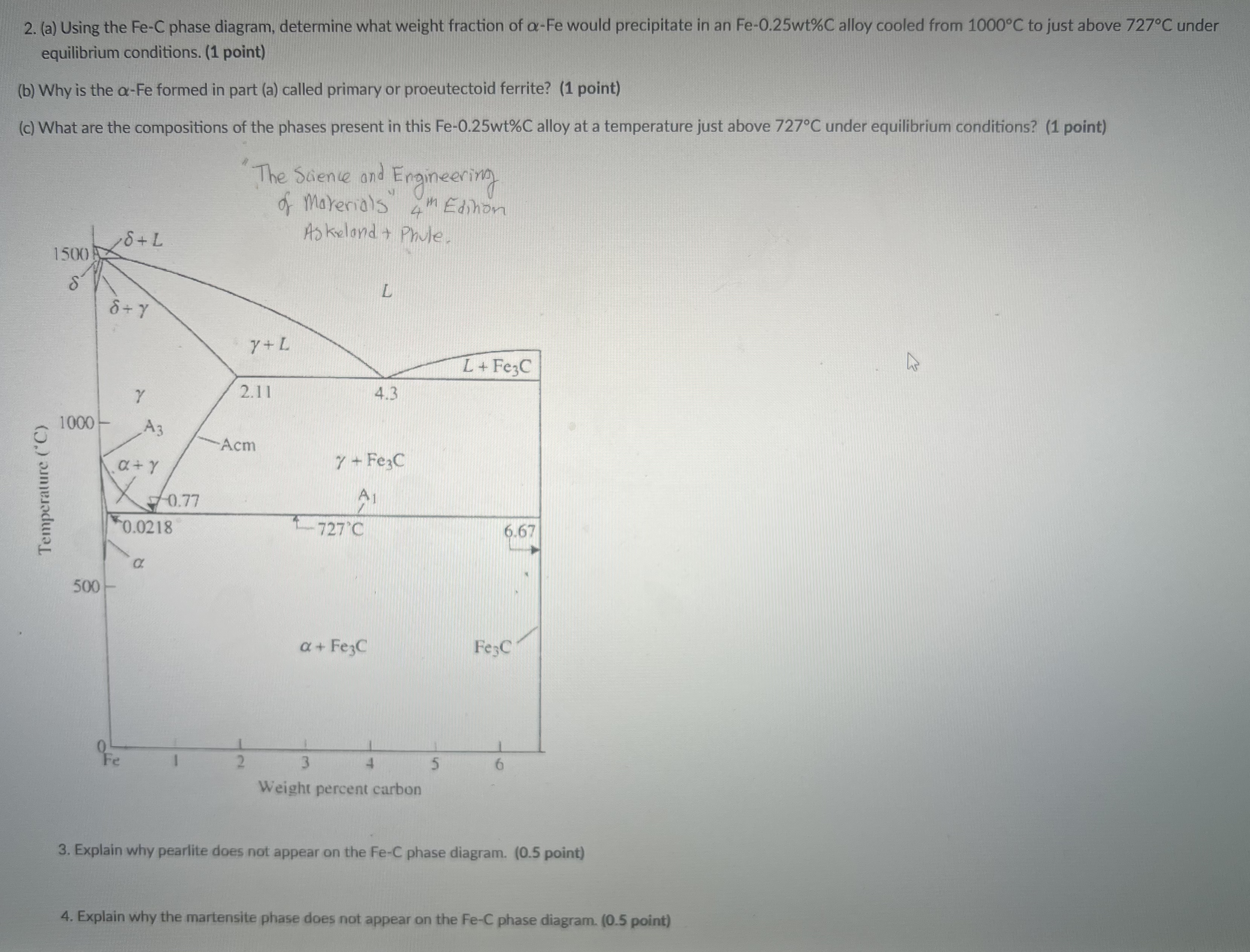
Question: ( a ) Using the F e - C phase diagram, determine what weight fraction of - Fe would precipitate in an F e -
a Using the phase diagram, determine what weight fraction of Fe would precipitate in an alloy cooled from to just above under equilibrium conditions.
b Why is the Fe formed in part a called primary or proeutectoid ferrite?
c What are the compositions of the phases present in this alloy at a temperature just above under equilibrium conditions?
Explain why pearlite does not appear on the FeC phase diagram.
Explain why the martensite phase does not appear on the phase diagram.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


