Question: a) Using the formula given below for the structure factor, determine the hkl reflections which are extincted in a FCC lattice. b) Determine the structure
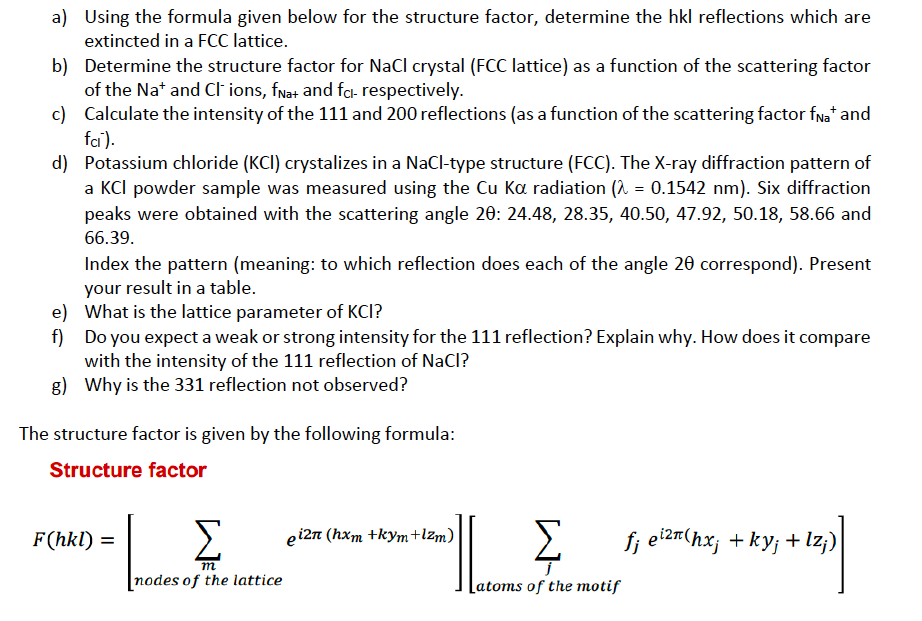
a) Using the formula given below for the structure factor, determine the hkl reflections which are extincted in a FCC lattice. b) Determine the structure factor for NaCl crystal (FCC lattice) as a function of the scattering factor of the Na+and Clions, fNa and fCl-. respectively. c) Calculate the intensity of the 111 and 200 reflections (as a function of the scattering factor fNa+and fCl) d) Potassium chloride ( KCl) crystalizes in a NaCl-type structure (FCC). The X-ray diffraction pattern of a KCl powder sample was measured using the CuK radiation (=0.1542nm). Six diffraction peaks were obtained with the scattering angle 2:24.48,28.35,40.50,47.92,50.18,58.66 and 66.39. Index the pattern (meaning: to which reflection does each of the angle 2 correspond). Present your result in a table. e) What is the lattice parameter of KCl ? f) Do you expect a weak or strong intensity for the 111 reflection? Explain why. How does it compare with the intensity of the 111 reflection of NaCl ? g) Why is the 331 reflection not observed? The structure factor is given by the following formula: Structure factor F(hkl)=[mnodesofthelatticeei2(hxm+kym+lzm)][jatomsofthemotiffjei2(hxj+kyj+lzj)]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


