Question: A vial containing an unknown sample melting at 133C is suspected to be one of the following compounds (mp.): urea (133C), 2,4-dimethylacetanilide (133C), phenacetin (134C)
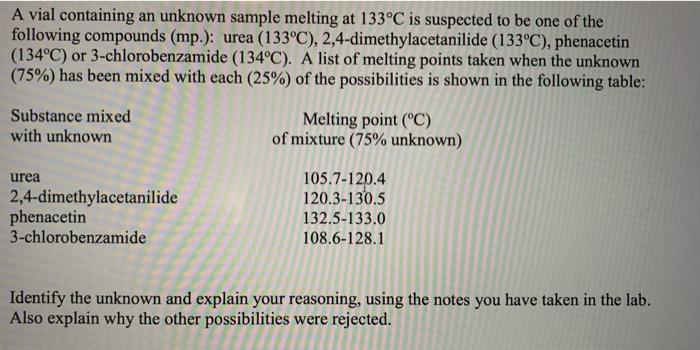
A vial containing an unknown sample melting at 133C is suspected to be one of the following compounds (mp.): urea (133C), 2,4-dimethylacetanilide (133C), phenacetin (134C) or 3-chlorobenzamide (134C). A list of melting points taken when the unknown (75%) has been mixed with each (25%) of the possibilities is shown in the following table: Substance mixed with unknown Melting point (C) of mixture (75% unknown) urea 2,4-dimethylacetanilide phenacetin 3-chlorobenzamide 105.7-120.4 120.3-130.5 132.5-133.0 108.6-128.1 Identify the unknown and explain your reasoning, using the notes you have taken in the lab. Also explain why the other possibilities were rejected
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


