Question: A voltaic cell is constructed from a standard H+H2 half cell (E red =0.000V) and a standard F2Fhalf cell (E red =2.870V). (Use the lowest
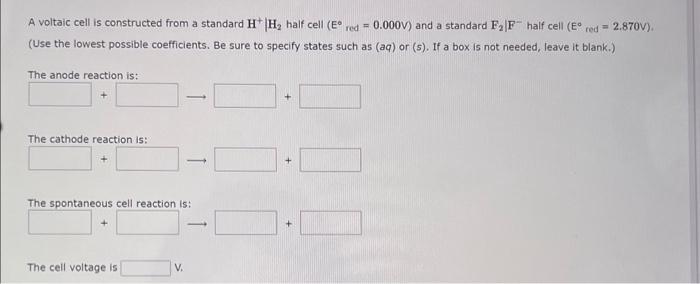
A voltaic cell is constructed from a standard H+H2 half cell (E red =0.000V) and a standard F2Fhalf cell (E red =2.870V). (Use the lowest possible coefficients. Be sure to specify states such as (aq) or ( 5 ). If a box is not needed, leave it blank.) The anode reaction is: The cathode reaction is: The spontaneous cell reaction is: The cell voltage is V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


