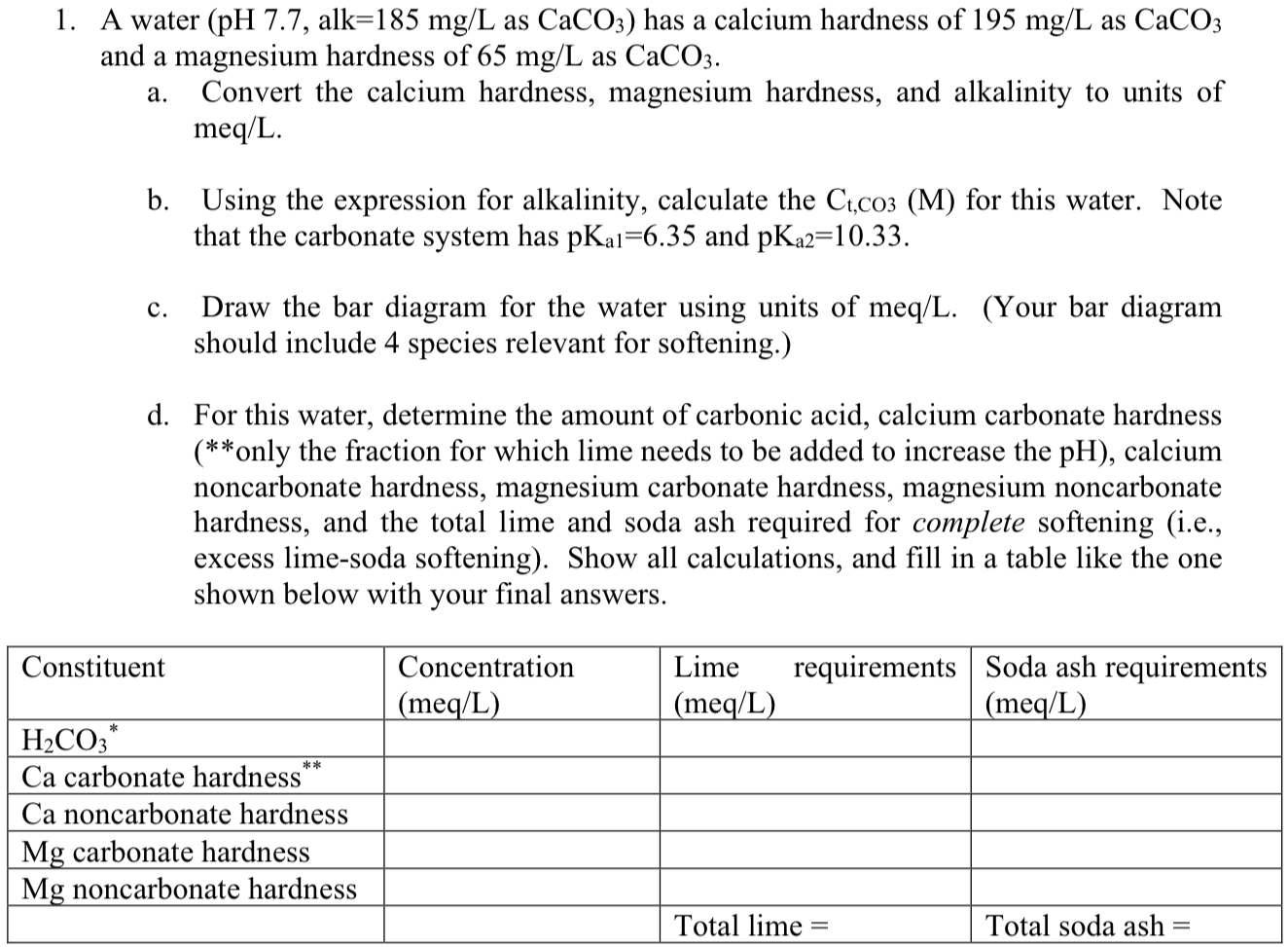
Question: A water , alk = 1 8 5 m g L as C a C O 3 ) has a calcium hardness of 1 9
A water alk as has a calcium hardness of as
and a magnesium hardness of as
a Convert the calcium hardness, magnesium hardness, and alkalinity to units of
b Using the expression for alkalinity, calculate the for this water. Note
that the carbonate system has and
c Draw the bar diagram for the water using units of meqLYour bar diagram
should include species relevant for softening.
d For this water, determine the amount of carbonic acid, calcium carbonate hardness
only the fraction for which lime needs to be added to increase the calcium
noncarbonate hardness, magnesium carbonate hardness, magnesium noncarbonate
hardness, and the total lime and soda ash required for complete softening ie
excess limesoda softening Show all calculations, and fill in a table like the one
shown below with your final answers.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


