Question: A water sample has the following analysis: amount Cation (mg/L) Ca+2 104 38 Mg+2 na* 19 Calculate the hardness provided by Mg+2 as mg/L of
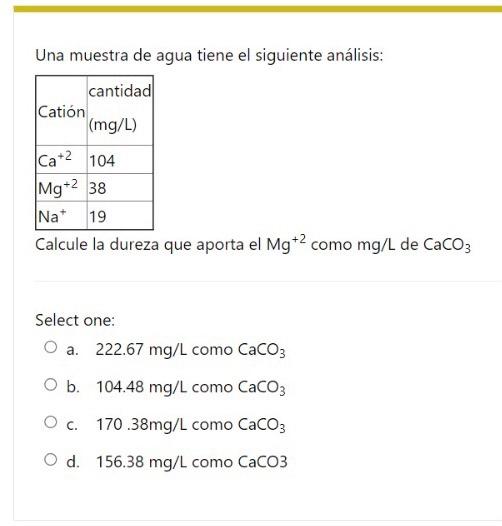
Una muestra de agua tiene el siguiente anlisis: Calcule la dureza que aporta el Mg+2 como mg/L de CaCO3 Select one: a. 222.67mg/L como CaCO3 b. 104.48mg/L como CaCO3 c. 170.38mg/L como CaCO3 d. 156.38mg/L como CaCO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


