Question: = = A water sample has the following composition: Na+ 120 ppm SO42- = 1110 ppm K+ HCO3- = 150 ppm SiO2 Temperature = 25C
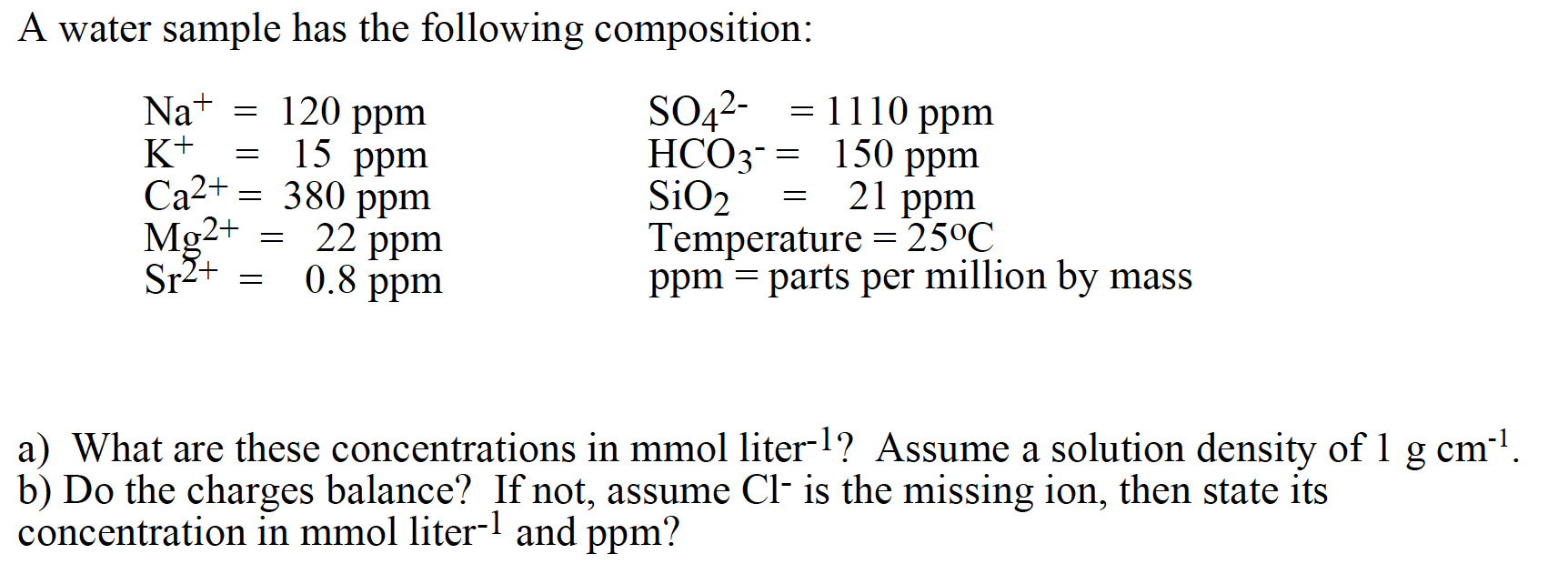
= = A water sample has the following composition: Na+ 120 ppm SO42- = 1110 ppm K+ HCO3- = 150 ppm SiO2 Temperature = 25C Sr2+ ppm = parts per million by mass = = = = = 15 ppm Ca2+ = 380 ppm 22 ppm 0.8 ppm 21 ppm Mg2+ - = = a) What are these concentrations in mmol liter-l? Assume a solution density of 1 g cm-1. b) Do the charges balance? If not, assume Cl- is the missing ion, then state its concentration in mmol liter-1 and ppm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


