Question: A water sample was analyzed and was found to have the following constituents (same as part 1): Cat?, mg/L Mg?, mg/L Na, mg/L Kt, mg/L
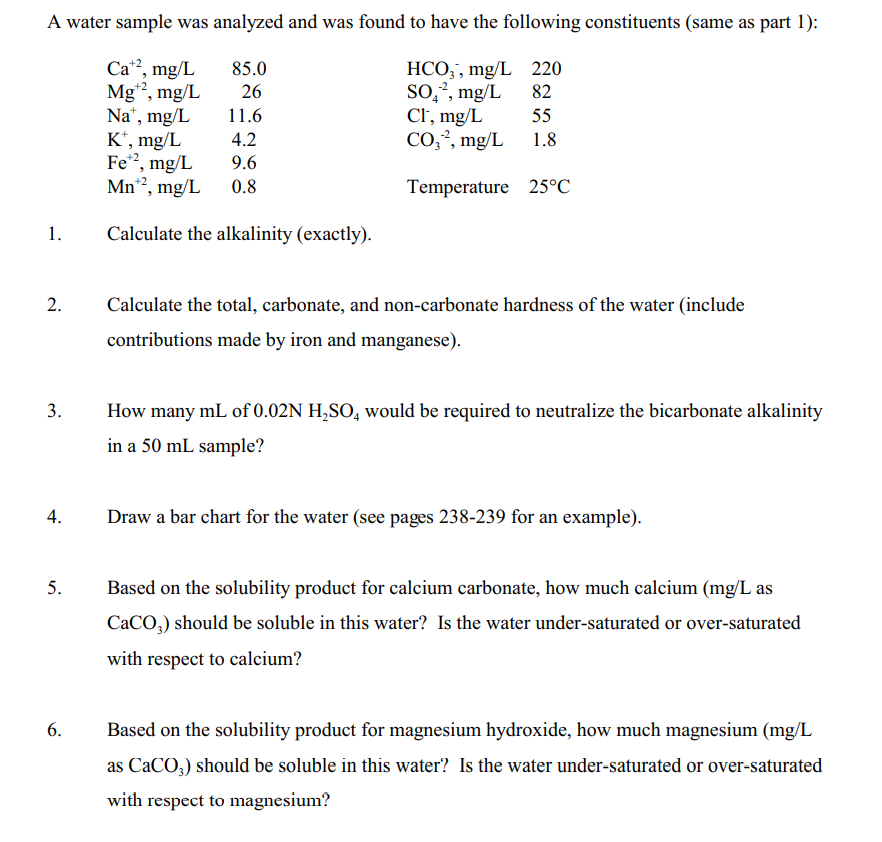
A water sample was analyzed and was found to have the following constituents (same as part 1): Cat?, mg/L Mg?, mg/L Na, mg/L Kt, mg/L Fe*?, mg/L Mn, mg/L 85.0 26 11.6 82 HCO., mg/L 220 SO,, mg/L Cl, mg/L 55 CO;?, mg/L 1.8 4.2 9.6 0.8 Temperature 25C 1. Calculate the alkalinity (exactly). 2. Calculate the total, carbonate, and non-carbonate hardness of the water (include contributions made by iron and manganese). 3. How many mL of 0.02N H,SO, would be required to neutralize the bicarbonate alkalinity in a 50 mL sample? 4. Draw a bar chart for the water (see pages 238-239 for an example). 5. Based on the solubility product for calcium carbonate, how much calcium (mg/L as CaCO3) should be soluble in this water? Is the water under-saturated or over-saturated with respect to calcium? 6. Based on the solubility product for magnesium hydroxide, how much magnesium (mg/L as CaCO3) should be soluble in this water? Is the water under-saturated or over-saturated with respect to magnesium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


