Question: ( a ) We have 5 0 0 same CSTRs in series, the reaction is 1 s t order liquid phase reaction. The volume for
a We have same CSTRs in series, the reaction is order liquid phase reaction. The volume for each reaction is with volumetric flow rate if we know the initial concentration of limiting reactant is mol please calculate the reaction rate at reactor. points
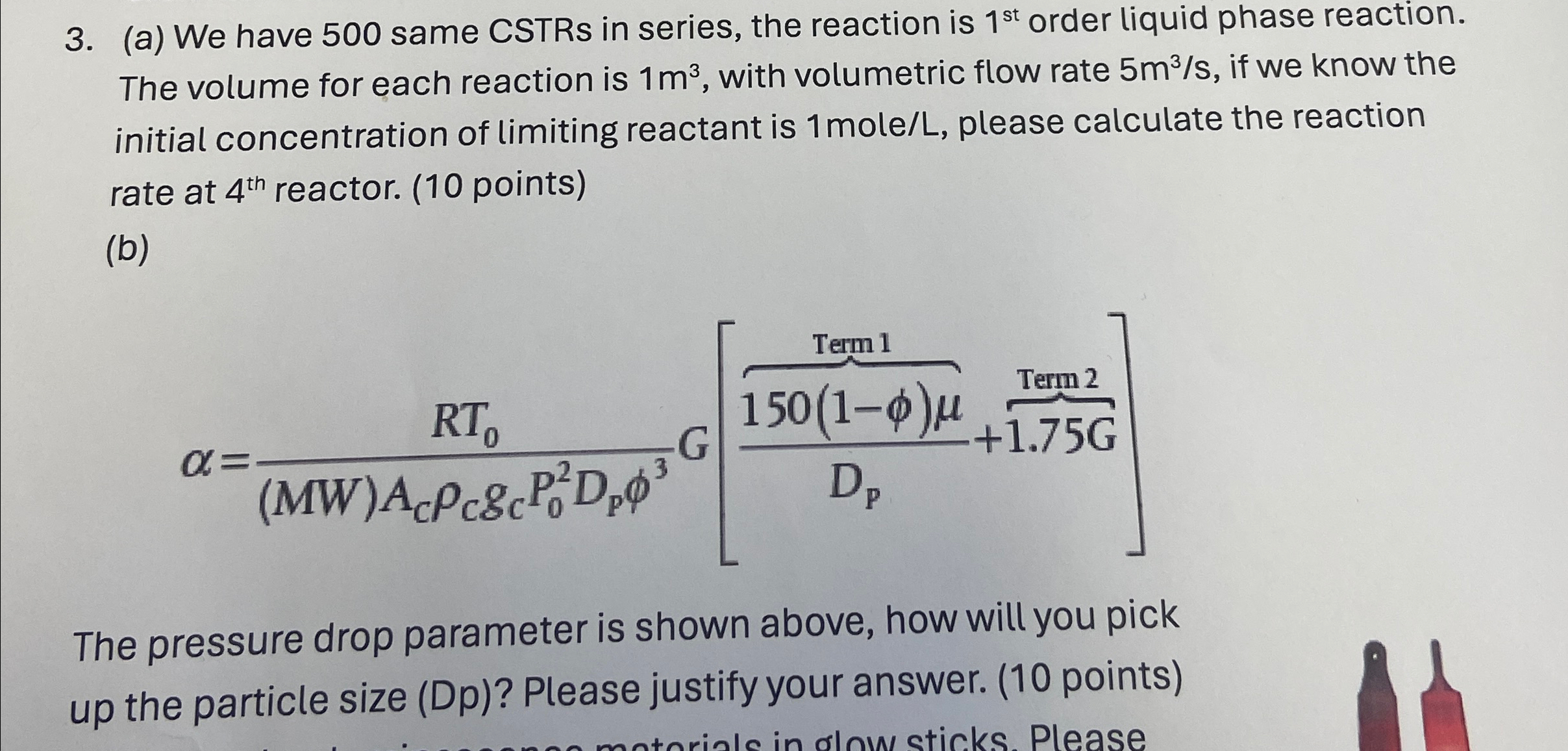
b
obrace
The pressure drop parameter is shown above, how will you pick up the particle size Dp Please justify your answer. points
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


