Question: A wet solid is dried from 25 to 10 per cent moisture under constant drying conditions in 15ks (4.17 h). If the critical and the

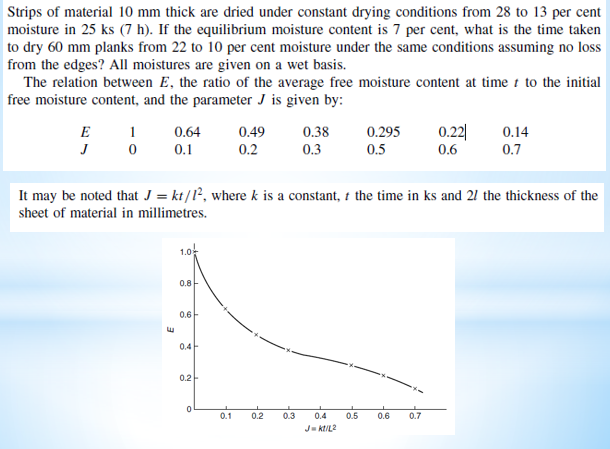


A wet solid is dried from 25 to 10 per cent moisture under constant drying conditions in 15ks (4.17 h). If the critical and the equilibrium moisture contents are 15 and 5 per cent respectively, how long will it take to dry the solid from 30 to 8 per cent moisture under the same conditions? Strips of material 10mm thick are dried under constant drying conditions from 28 to 13 per cent moisture in 25ks(7h). If the equilibrium moisture content is 7 per cent, what is the time taken to dry 60mm planks from 22 to 10 per cent moisture under the same conditions assuming no loss from the edges? All moistures are given on a wet basis. The relation between E, the ratio of the average free moisture content at time t to the initial free moisture content, and the parameter J is given by: It may be noted that J=kt/l2, where k is a constant, t the time in ks and 2l the thickness of the sheet of material in millimetres. A 100kg batch of granular solids containing 30 per cent moisture is to be dried in a tray drier to 15.5 per cent of moisture by passing a current of air at 350K tangentially across its surface at a velocity of 1.8m/s. If the constant rate of drying under these conditions is 0.0007kg/sm2 and the critical moisture content is 15 per cent, calculate the approximate drying time. Assume the drying surface to be 0.03m2/kg dry mass. A wet solid is dried from 35 to 10 per cent moisture under constant drying conditions in 18ks(5h). If the equilibrium moisture content is 4 per cent and the critical moisture content is 14 per cent, how long will it take to dry to 6 per cent moisture under the same conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


